Paramedische richtlijn parkinson met beslisondersteuning
B.3.2 Optionele meetinstrumenten
Naast de aanbevolen meetinstrumenten heeft de werkgroep optionele meetinstrumenten geselecteerd die toegepast kunnen worden bij patiënten met de ziekte van Parkinson.
Overzicht van de optionele meetinstrumenten (www.meetinstrumentenzorg.nl).
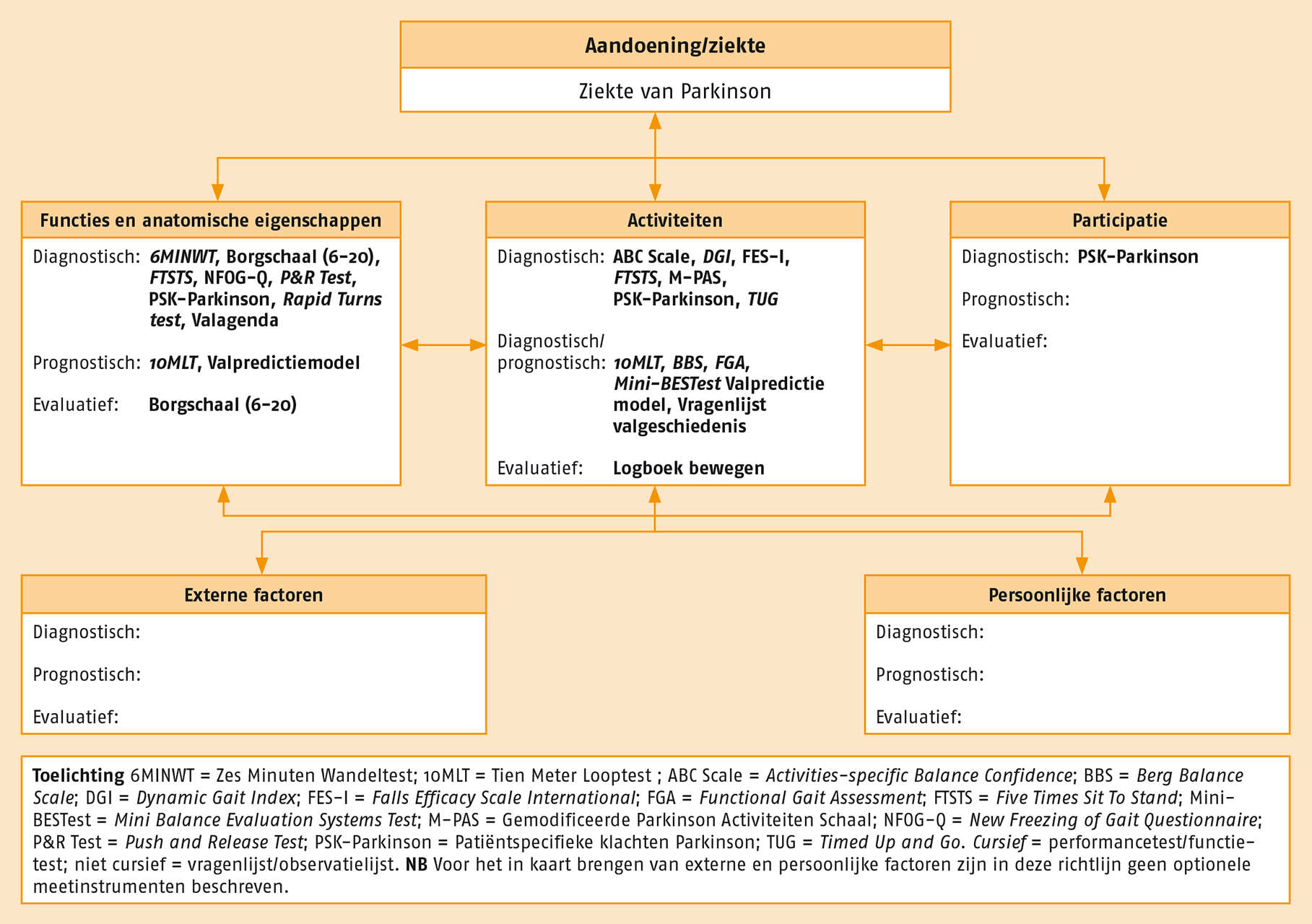
6MINWT = Zes Minuten Wandeltest; 10MLT = Tien Meter Looptest ; ABC Scale = Activities-specific Balance Confidence; BBS = Berg Balance Scale; DGI = Dynamic Gait Index; FES-I= Falls Efficacy Scale International; FGA = Functional Gait Assessment; FTSTS = Five Times Sit To Stand; Mini-BESTest = Mini Balance Evaluation Systems Test; M-PAS = Gemodificeerde Parkinson Activiteiten Schaal; NFOG-Q = New Freezing of Gait Questionnaire; P&R Test = Push and Release Test; PSK-Parkinson = Patiëntspecifieke klachten Parkinson; TUG = Timed Up and Go; Valagenda, Vragenlijst valgeschiedenis.
Cursief = performancetest/functietest; niet cursief = vragenlijst/observatielijst.
In de volgende paragrafen wordt – in alfabetische volgorde – kort aangehaald welke aandachtspunten er zijn bij de ziekte van Parkinson en, indien van toepassing, wat de toegevoegde waarde van het instrument is ten opzichte van andere instrumenten.
Zes Minuten Wandeltest (6MINWT)
Aandachtsgebieden: fysieke capaciteit en lopen
De 6MINWT is geschikt om op een objectieve manier de submaximale functionele inspannings-capaciteit te beoordelen en te evalueren en tevens is de test geschikt voor loopobservatie gedurende langere tijd.335-337 De werkgroep raadt aan om bij het afnemen van de 6MINWT ook de Borgschaal 6-20 te gebruiken om direct inzicht te verkrijgen in de door de patiënt ervaren inspanning.338,339
Het gebruik van een loopband is niet aan te bevelen voor de 6MINWT, omdat patiënten op een loopband niet goed hun eigen tempo kunnen bepalen.336
De werkgroep raadt aan de 6MINWT te gebruiken als uit de Intakevragenlijst blijkt dat de persoon weinig actief is. Als de patiënt tijdens de 6MINWT maar een korte afstand kan afleggen, is verder onderzoek nodig naar de oorzaak van de stoornis.
Daarbij dient ook gekeken te worden naar de spiersterkte. Het kan in dat geval ook zinvol zijn om de longfunctie, hartfunctie, enkel-armindex, voedingstoestand en cognitieve functie en het functioneren van het steun- en bewegingsapparaat te controleren.336 Als de 6MINWT gebruikt wordt om veranderingen in het uithoudingsvermogen bij te houden, dient daarbij de absolute verandering in meters te worden geregistreerd.
Tien Meter Looptest (10MLT)
Aandachtsgebied: lopen
Loopsnelheid is van belang voor de veiligheid, bijvoorbeeld bij het oversteken van de straat. Met de 10MLT kan de loopsnelheid bij zowel een comfortabel als een hoog looptempo beoordeeld en geëvalueerd worden.111 Deze test is ook geschikt voor het bepalen van de schredelengte en de cadans (stapfrequentie).
De schredelengte is van belang als het gebruik van visuele cues overwogen wordt en de cadans is van belang als het gebruik van auditieve cues overwogen wordt (paragraaf C.2.2). Patiënten mogen bij deze test een loophulpmiddel gebruiken. De 10MLT is niet gevalideerd om lopen met een dubbeltaak te evalueren, maar dubbeltaken gebruiken tijdens de 10MLT helpt wel om vast te stellen of een patiënt een verhoogd valrisico heeft.
Bij ruimtegebrek kan de 10MLT worden ingekort tot 6 meter (6MLT). De 6MLT is echter niet gevalideerd voor het meten van veranderingen.
Activities-specific Balance Confidence (ABC) Scale
Aandachtsgebied: balans
Op de ABC Scale geven patiënten voor verschillende activiteiten aan hoeveel vertrouwen ze hebben dat ze die activiteit kunnen uitvoeren zonder hun balans te verliezen.340
De ABC Scale helpt bij het vaststellen van een verhoogd valrisico341 en kan ook gebruikt worden om eventuele verandering vast te stellen. Daarnaast is de score op de ABC Scale een bepalende factor voor de functionele wandelcapaciteit, die gemeten wordt met de 6MINWT.255
Berg Balance Scale (BBS)
Aandachtsgebied: balans
De BBS beoordeelt beperkingen in activiteiten bij het uitvoeren van functionele taken die een beroep op de balans doen.342 De BBS helpt bij het vaststellen of een patiënt een verhoogd valrisico heeft. Een nadeel van de BBS is dat veel patiënten met de ziekte van Parkinson de maximale score behalen. Een mogelijke verklaring voor dit plafondeffect is dat de BBS freezing en parkinsonspecifieke beperkingen in activiteiten bij het uitvoeren van dubbeltaken niet betrekt bij beoordeling.
Ook wordt het lopen niet beoordeeld. De werkgroep raadt daarom aan de BBS alleen te gebruiken bij patiënten in een gevorderd stadium van de ziekte, die minder mobiel zijn en vooral in stilstand balansproblemen hebben.
Borgschaal 6-20
Aandachtsgebied: fysieke capaciteit
Met de Borgschaal 6-20 kan inzicht verkregen worden in de ervaren inspanning.338 Dit meetinstrument geeft bij gezonde mensen van middelbare leeftijd en ouderen een goed beeld van de inspanningsintensiteit en laat bij deze populaties een goede correlatie zien met fysiologische criteria, zoals de hartslag.339,343
De validiteit, betrouwbaarheid en hanteerbaarheid van de Borgschaal 6-20 zijn niet apart onderzocht bij patiënten met de ziekte van Parkinson, maar volgens de werkgroep is er geen reden om dit meetinstrument niet te gebruiken.
Dynamic Gait Index (DGI) en de Functional Gait Assessment (FGA)
Aandachtsgebied: balans
Met de DGI en de FGA kan zowel de statische als de dynamische balans beoordeeld worden. Beide tests beschikken over goede psychometrische kenmerken, zijn goed toepasbaar en helpen achterhalen of de patiënt een verhoogd valrisico heeft. Voor de DGI is zelfs een afkapwaarde vastgesteld voor de aanwezigheid van een verhoogd valrisico en dit meetinstrument kan gebruikt worden om eventuele veranderingen bij te houden.
Bij de DGI wordt een score gegeven voor de balans tijdens het uitvoeren van acht loopactiviteiten.344,345 De scores voor afzonderlijke testitems helpen bij het vaststellen van onderliggende stoornissen, zoals gebrekkige dynamische controle van het lichaamszwaartepunt of afwijkende gewichtsverdeling. Deze informatie is van belang bij het bepalen van de behandeldoelen en het kiezen van een interventie.
De FGA wordt gezien als een verbeterde versie van de DGI. De FGA neemt ook het achteruitlopen mee in de beoordeling, maar vereist wel een tijdrovende voorbereidingsprocedure, omdat er eerst tapemarkeringen moeten worden aangebracht.
Aangezien de FGA en de DGI veel vergelijkbare items bevatten, kunnen ze gecombineerd worden. De FGA bevat drie extra activiteiten en geeft daarmee extra duidelijkheid over het al dan niet aanwezig zijn van een verhoogd valrisico.345 Vooral het achteruitlopen levert waardevolle informatie op over de balans bij stoeltransfers. De werkgroep adviseert om de tests tijdens een off-fase uit te voeren om nauwkeuriger te kunnen vaststellen of er sprake is van een verhoogd valrisico.346
Falls Efficacy Scale International (FES-I)
Aandachtsgebied: balans
Bij minder mobiele patiënten kan overwogen worden om in plaats van de ABC Scale de FES-I te gebruiken.347 Als de fysiotherapeut maar weinig tijd heeft, kan gekozen worden voor de verkorte versie, de Short FES-I (de items 2, 4, 6, 7, 9, 15 en 16 van de oorspronkelijke FES-I).
De FES-I geeft echter meer inzicht in de angst om te vallen tijdens binnen- en buitenactiviteiten dan de Short FES-I en levert dus meer informatie op ten aanzien van behandeldoelen en de meest geschikte interventie. Bovendien is er maar weinig bekend over de psychometrische kenmerken van het gebruik van de FES-I bij parkinson.
Five Times Sit to Stand (FTSTS)
Aandachtsgebieden: fysieke capaciteit en transfers
De FTSTS is een snel uit te voeren test van de functionele bewegingen en helpt bij het vaststellen van onvoldoende beenspiersterkte en -uithoudingsvermogen. Tevens is de test geschikt om de balans te testen bij een stoelgerelateerde transfer en om te beoordelen of de patiënt een verhoogd valrisico heeft.348
Bij deze test wordt bekeken in hoeveel tijd de patiënt kan opstaan uit een 43 cm hoge stoel. De werkgroep raadt het gebruik van de FTSTS, in combinatie met de Push and Release (P&R) Test aan voor patiënten bij wie onzekerheid bestaat over hun balans bij transfers. De FTSTS levert geen gedetailleerde informatie op over balansproblemen tijdens het lopen en in stilstand.
Gemodificeerde Parkinson Activiteiten Schaal (M-PAS)
Aandachtsgebieden: transfers, balans en lopen
De Gemodificeerde M-PAS beoordeelt de kwaliteit van en de beperkingen in functionele bewegingen.349 De M-PAS test drie functionele bewegingen: ‘transfer stoel’, ‘gangakinesie’ en ‘bedmobiliteit’. In het onderdeel Gangakinesie wordt ook de Timed Up and Go (TUG) uitgevoerd. Deze test kan gedaan worden met een motorische dubbeltaak (al lopend een dienblad met bekers water dragen) of met een cognitieve dubbeltaak (al lopend terugtellen).
Door bij te houden hoeveel tijd de activiteit kost, kan de TUG ook daadwerkelijk gescoord worden (het is dan van belang om de ‘U’ op 3 meter te vervangen door een pilon (immers zonder de draai in een ‘nieuwe U’ zullen sommige patiënten sneller lopen en is het niet zuiver de TUG).
Het is belangrijk dat de patiënt elke keer hetzelfde schoeisel draagt.350 De bedmobiliteit wordt met en zonder gebruik van een deken getest. De M-PAS levert belangrijke informatie op bij het bepalen van de behandeldoelen en het kiezen van een interventie.
Welke onderdelen van de test gebruikt worden, hangt af van de ervaren problemen.
- Voor beoordeling van de balans worden de onderdelenTransfer stoel en Gangakinesie aanbevolen;
- ter beoordeling van de kwaliteit van stoel- of bedgerelateerde transfers raadt de werkgroep de onderdelen Transfer stoel en Bedmobiliteit aan;
- ter beoordeling van de loopkwaliteit wordt het onderdeel Gangakinesie aangeraden.
Logboek bewegen
Aandachtsgebied: fysieke capaciteit
In het Logboek bewegen houdt de patiënt een week bij hoeveel minuten hij aan bepaalde activiteiten besteedt. Tevens kan de mate van inspanning middels de Borgschaal genoteerd worden.338 Het Logboek bewegen kan ter inventarisatie en ter evaluatie worden gebruikt.
Mini Balance Evaluation Systems Test (Mini-BESTest)
Aandachtsgebied: balans
Met de Mini-BESTest kan zowel de statische als de dynamische balans beoordeeld worden.351Enkele testonderdelen zijn de TUG, de Push and Release (P&R) Test en de loopkwaliteit tijdens het veranderen van loopsnelheid, het ontwijken van obstakels en het draaien om de as.
De Mini-BESTest beschikt over goede psychometrische kenmerken, is goed toepasbaar en is te gebruiken om veranderingen bij te houden.352 Voor de Mini-BESTest is tevens een afkapwaarde vastgesteld voor de aanwezigheid van een verhoogd valrisico.
Nine-hole Peg Test
Aandachtsgebied: arm- en handvaardigheid
Voor het beoordelen van het dragen, verplaatsen of vastpakken kunnen geen specifieke meetinstrumenten worden aanbevolen. Het enige meetinstrument op dit gebied waarvan de validiteit, betrouwbaarheid en responsiviteit gevalideerd zijn voor gebruik bij parkinson is de Nine-hole Peg Test.353
Hiermee worden nauwkeurige hand- en vingerbewegingen beoordeeld. Deze test kan gebruikt worden ter evaluatie van training van de fijne motoriek van de handen. De Nine-hole Peg Test geeft echter geen inzicht in de kwaliteit van de uitvoering en in de aandachtsgebieden voor fysiotherapeutische interventie.
Bovendien moet voor het gebruik van deze test betaald worden. De werkgroep raadt daarom aan om deze test alleen te gebruiken als die al voorhanden is op de behandellocatie (ziekenhuizen met een afdeling ergotherapie beschikken vaak over deze test).
Push and Release (P&R) Test
Aandachtsgebied: balans
De P&R Test beoordeelt de balanscontrole tijdens het stilstaan.354 Deze test geeft inzicht in onwillekeurige bewegingsreacties die een rol spelen bij het bewaren van de balans wanneer de patiënt achteruitloopt (bijvoorbeeld bij het openen van een deur of het gaan zitten) en bij lopen op een gladde ondergrond. De retropulsietest (opgenomen in de Unified Parkinson’s Disease Rating Scale van de Movement Disorder Society, de MDS-UPDRS323) is onder neurologen en fysiotherapeuten bekender.
Bij die test worden balansproblemen vastgesteld door te controleren hoe goed patiënten hun balans kunnen hervinden na een plotselinge achterwaartse ruk. De P&R Test is echter gevoeliger tijdens off-fasen en beter toepasbaar bij kwetsbare patiënten en beschikt daarnaast over een betere indruksvaliditeit voor gebruik binnen de fysiotherapeutische zorg.
Rapid Turns test
Aandachtsgebied: balans
Voor patiënten die op de Intakevragenlijst hebben aangegeven last te hebben van freezing, is het zinvol om naast de NFOG-Q, de Rapid Turns Test te gebruiken. Deze test lokt freezing uit.272 De patiënt wordt gevraagd om herhaaldelijk helemaal om zijn as te draaien, in beide richtingen, met hoge snelheid en een kleine draaicirkel.272 Als het niet lukt om op deze manier freezing uit te lokken, kunnen er dubbeltaken worden toegevoegd. Vaak is het lastig om festinatiestappen die aan freezing voorafgaan te onderscheiden van pure festinatie, die niet door freezing wordt gevolgd.319
Om vast te stellen of de patiënt vrijwillig stopt of last heeft van freezing, beoordeelt de fysiotherapeut of de volgende kenmerken van freezing aanwezig zijn:319
- een gebogen houding met vaste flexie in de heup-, knie- en enkelgewrichten;
- onvolledig tot stilstand komen, waarbij de patiënt op de plaats natrilt of schuifelpasjes naar voren maakt;
- in veel gevallen: voorafgaand aan de freezing steeds kleiner wordende stappen en een toenemende cadans;
- het gevoel hebben aan de grond genageld te staan.
Timed Up and Go (TUG)
Aandachtsgebieden: transfers, balans en lopen
De TUG is een korte test die een vermogensmaat voor functionele bewegingen oplevert.355 De TUG is geschikt om loopsnelheid in het kader van functionele bewegingen te beoordelen. Daarnaast helpt de TUG om een eventueel valrisico vast te stellen (hoe langer de duur, hoe groter de kans op een verhoogd valrisico).
Om nauwkeuriger te kunnen vaststellen of iemand een verhoogd valrisico heeft, adviseert de werkgroep de TUG tijdens de off-fase uit te voeren.346 De werkgroep raadt bovendien aan om niet alleen de benodigde tijd te beoordelen, maar ook te noteren hoe veilig de persoon zich omdraait.
NB De TUG kan gecombineerd worden met de Gemodificeerde Parkinson Activiteiten Schaal (M-PAS) (in het onderdeel Gangakinesie) en de Balance Evaluation Systems Test (Mini-BESTest).
Valagenda
Aandachtsgebied: balans
De Valagenda is bedoeld om inzicht te krijgen in de valfrequentie, de omstandigheden waaronder de valincidenten plaatsvinden en de mogelijke oorzaken ervan en wordt aanbevolen voor gebruik in de klinische praktijk.356 De werkgroep raadt fysiotherapeuten aan om de Valagenda mee te geven aan patiënten die vermoedelijk een verhoogd valrisico hebben. Indien van toepassing, dient de Valagenda samen met de mantelzorger te worden ingevuld.
Valpredictiemodel
Aandachtsgebied: balans
Of een op valpreventie gerichte interventie nodig is, is goed vast te stellen aan de hand van gegevens over vallen, freezing en de comfortabele loopsnelheid. Met het Valpredictiemodel wordt beoordeeld of de patiënt een laag, matig of hoog risico heeft om binnen zes maanden na afname van de test te vallen (met respectievelijk de volgende absolute risico’s: 17%, 51% en 85%).269 Deze uitkomst helpt bepalen of er een indicatie is voor interdisciplinaire beoordeling, individuele fysiotherapeutische behandeling of dat deelname aan een algemene beweeggroep mogelijk is.
De werkgroep wil benadrukken dat bij de besluitvorming niet alleen naar de afkapscore gekeken moet worden, maar juist naar het complete klinische beeld:
- eventuele aanwezigheid van freezing;269,277,357
- eventuele aanwezigheid van dementie;233,358
- eventuele aanwezigheid van (lichte) cognitieve stoornissen, bijvoorbeeld in het werkgeheugen en de responsgeneratie;359,360
- eventuele aanwezigheid van urine-incontinentie;256
- eventuele verminderde armzwaai;233
- het item van de UPDRS dat is gericht op snel wisselende taken;361
- eventuele verminderde aandacht en executieve functies;362,363
- het aantal jaren sinds diagnosticering;233
- externe factoren, zoals te veel meubelen in huis, gladde vloer, losliggend tapijt, slechte verlichting en ongeschikt schoeisel;
- eventuele bijwerkingen van medicatie (Addendum Praktijk, hoofdstuk 3), zoals hallucinaties;
- eventuele comorbiditeiten, zoals diabetische neuropathie;
- de frequentie en veiligheid van alledaagse activiteiten (zoals multitasken).
Afkapscores zeggen meestal iets over het valrisico voor de komende drie tot zes maanden.364
De gevoeligheid van een afkapscore geeft aan hoeveel procent van de patiënten met een verhoogd valrisico, ook daadwerkelijk als zodanig wordt aangewezen door het betreffende meetinstrument. Dus hoe hoger het gevoeligheidspercentage van de afkapscore, hoe groter de kans dat het betreffende meetinstrument bij een patiënt terecht een verhoogd valrisico vaststelt.
De kans dat terecht een verhoogd valrisico wordt vastgesteld, is bij een patiënt met een score op de Activities-specific Balance Confidence (ABC) Scale van 65% bijvoorbeeld groter dan bij een patiënt die in het voorgaande jaar één keer gevallen is, aangezien de gevoeligheid bij deze bevindingen respectievelijk 93% en 77% is.
Vaak worden bij een patiënt meerdere meetinstrumenten gebruikt. Als de scores van meerdere meetinstrumenten boven de bijbehorende afkapwaarde vallen, zal de gevoeligheid waarschijnlijk nog hoger zijn dan in de tabel vermeld wordt voor de afzonderlijke scores. De afkapscores kunnen dus een waardevol hulpmiddel zijn in de klinische praktijk. In de volgende tabel staat van verschillende aanbevolen en optionele meetinstrumenten de afkapscore.
Afkapscores die inzicht geven in het valrisico bij de ziekte van Parkinson.
| Meetinstrument | Aantal patiënten | H&Y-score, spreiding/gemiddelde | Afkapscore | Gevoeligheid (%) AUC of OR |
| Valgeschiedenis (voorgaande 12 maanden) | 349 | 1-5 / 2,4 | ≥ 1 valincident | 77%232 |
| ABC Scale | 20 | 2,9 / ? | < 69% | 93%253 |
| DGI | 45 | 2-3 / 2,6 | ≤ 22 | 89%365 |
| FGA | 80 | 1-4 / 2,5 | ≤ 15/30 | 72%, AUC 0,80367 |
| BBS | 49 | 2-3 / ? | < 44 | 68%, AUC 0,85, OR 48,9341 |
| Mini-BESTest | 80b | 1-4 | < 20 | 88%368 |
| FTSTS | 82 | 1-4 / 2,4 | > 16 sec | 75%, AUC 0,77371 |
| TUG | 45 | 2-3 / 2,6 | ≥ 7,95 sec | 93%365 |
| 10MLTd | 78 | 1-4 / 2,6 | < 0,98 m/sec | 80%; AUC 0,80268 |
| Valpredictiemodel | 205 | 1-4 / 2,6 | 10MLTa <1,1 m/sec en ≥ 1 valincident/12 mnd | AUC 0,80 (95%-BI 0,73-0,86)a, 269 |
| 10MLT = Tien Meter Looptest; ABC Scale = Activities-specific Balance Confi dence Scale; BBS = Berg Balance Scale; DGI = Dynamic Gait Index; FGA = Functional Gait Assessment; FTSTS = Five Times Sit To Stand; Mini-BESTest = Mini Balance Evaluation Systems Test; TUG = Timed Up and Go. H&Y = Hoehn en Yahr. ? = onbekend. AUC = area under the curve: een AUC-waarde > 0,70 voldoet; OR = oddsratio: hoeveel keer groter de kans is dat patiënten met een score boven de afkapwaarde terecht als ‘vallers’ worden aangemerkt. a Voorspellend voor de komende zes maanden. b n = 29 niet beschikbaar voor follow-up. | ||||
1. Wees PJ van der, Hendrik s HJM, Heldoorn M, Custers JWH, Bie RA de. Methode voor ontwikkeling, implementatie en bijstelling van KNGF-richtlijnen. Amersfoort/Maastricht. 2007.
2. Vries C de, Hagenaars L, Kiers H, Schmitt M. Amersfoort: KNGF Beroepsprofiel Fysiotherapeut; 2013.
3. Bergen JL, Toole T, Elliott III RG, Wallace B, Robinson K, Maitland CG. Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson’s disease patients. Neuro Rehabil. 2002;17(2):161-8.
4. Blackinton MJ, Summerall L, Waguespack K. Tertiary prevention in Parkinson disease: Results from a preliminary study. Neurol Report. 2002;26(160):165.
5. Bridgewater KJ, Sharpe M. Aerobic exercise and early Parkinson’s disease. Neurorehabil Neural Repair. 1996;10:233-41.
6. Burini D, Farabollini B, Iacucci S, Rimatori C, Riccardi G, Capecci M, et al. A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson’s disease. Eura Medicophys. 2006;42(3):231-8.
7. Byl N. Enhancing safe mobility in patients with Parkinson’s disease: effect of dual task training during aerobic and moderate exercise. Parkinsonism Relat Disord. 2009;15 (Suppl 2):S122.
8. Cerri C, Arosio A, Biella AM, Premoselli S, Piccini L. Physical exercise therapy of Parkinson’s. Mov Disord. 1994;9 (suppl.1):68.
9. Chiviacowsky S, Wulf G, Lewthwaite R, Campos T. Motor learning benefits of self-controlled practice in persons with Parkinson’s disease. Gait Posture. 2012;35(4):601-5.
10. Chouza M, Arias P, Vinas S, Cudeiro J. Acute effects of whole-body vibration at 3, 6, and 9 hz on balance and gait in patients with Parkinson’s disease. Mov Disord. 2011;26(5):920-1.
11. Cianci H, Robinson K, Bunting-Perry L, Sollenberger J, Noorigian J, Duda J. Are wheeled walkers with visual cues efficacious to treat freezing of gait in Parkinson’s disease? Parkinsonism Relat Disord. 2010;16 (Suppl 1):S64.
12. Dam M, Tonin P, Casson S, Bracco F, Piron L, Pizzolato G, et al. Effects of conventional and sensory-enhanced physiotherapy on disability of Parkinson’s disease patients. Adv Neurol. 1996;69:551-5.
13. Fiorani C, Mari F, Bartolini M, Ceravolo MG, Provinciali L. Occupational therapy increases ADL score and quality of life in Parkinsonian patients. Mov Disord. 1997;12(S1):135.
14. Fok P, Farrell M, McMeeken J. The effect of dividing attention between walking and auxiliary tasks in people with Parkinson’s disease. Hum Mov Sci. 2012;31(1):236-46.
15. Forkink A, Toole T, Hirsch MA, Lehman DA, Maitland CG. The effects of a balance and strengthening program on equilibrium in Parkinsonism. Working Paper Series. 1996;PI-96-33.
16. Formisano R, Pratesi L, Modarelli FT, Bonifati V, Meco G. Rehabilitation and Parkinson’s disease. Scand J Rehabil Med. 1992;24(3):157-60.
17. Ganesan M, Pal PK, Gupta A, Talakad S. Effect of partial weight supported treadmill gait training on balance in patients of Parkinson’s disease. Mov Disord. 2010;16 (Suppl 1):S66.
18. Gauthier L, Dalziel S, Gauthier S. The benefits of group occupational therapy for patients with Parkinson’s disease. Am J Occup Ther. 1987;41(6):360-5.
19. Gibberd FB, Page NG, Spencer KM, Kinnear E, Hawksworth JB. Controlled trial of physiotherapy and occupational therapy for Parkinson’s disease. Br Med J (ClinResEd). 1981;282(6271):1196.
20. Gobbi LT, Oliveira-Ferreira MD, Caetano MJ, Lirani-Silva E, Barbieri FA, Stella F, et al. Exercise programs improve mobility and balance in people with Parkinson’s disease. Parkinsonism Relat Disord. 2009;15 Suppl 3:S49-S52.
21. Goodwin V, Richards S, Ewings P, Taylor A, Campbell J. Preventing falls in Parkinson’s disease: the GETuP trial. Parkinsonism Rel Disord. 2009;15 (Suppl 2):S49.
22. Guo L, Jiang Y, Yatsuya H, Yoshida Y, Sakamoto J. Group education with personal rehabilitation for idiopathic Parkinson’s disease. Can J Neurol Sci. 2009;36(1):51-9.
23. Haas CT, Turbanski S, Kessler K, Schmidtbleicher D. The effects of random whole-body-vibration on motor symptoms in Parkinson’s disease. Neuro Rehabil. 2006;21(1):29-36.
24. Hackney ME, Earhart GM. Health-related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism Relat Disord. 2009.
25. Hass CJ, Waddell DE, Wolf SL, Juncos JL, Gregor RJ. The influence of tai chi training on locomotor ability in Parkinson’s disease. Proceed Ann Meeting Am Spc Biomechan. 2006;21.
26. Hass CJ, Collins MA, Juncos JL. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: a randomized trial. Neurorehabil Neural Repair. 2007;21(2):107-15.
27. Homann CN, Crevenna R, Koinig H, Kurzl B, Reinprecht S, Wenzel K, et al. Can physiotherapy improve axial symptoms in parkinsonian patients? A pilot study with the computerized movement analysis battery Zebris. Mov Disord. 1998;13 (Suppl 2):234.
28. Hurwitz A. The benefit of a home exercise regimen for ambulatory Parkinson’s disease patients. J Neurosc iNurs. 1989;21(3):180-4.29. Inzelberg R, Peleg N, Nisipeanu P, Magadle R, Carasso RL, Weiner P. Inspiratory muscle training and the perception of dyspnea in Parkinson’s disease. Can J Neurol Sci. 2005;32(2):213-7.
30. Katsikitis M, Pilowsky I. A controlled study of facial mobility treatment in Parkinson’s disease. J Psychosom Res. 1996;40(4):387-96.
31. King LK, Almeida QJ, Ahonen H. Short-term effects of vibration therapy on motor impairments in Parkinson’s disease. Neuro Rehabil. 2009;25(4):297-306.
32. Lee KS, Lee WH, Hwang S. Modified constraint-induced movement therapy improves fine and gross motor performance of the upper limb in Parkinson disease. Am J Phys Med Rehabil. 2011;90(5):380-6.
33. Lehman DA, Toole T, Lofald D, Hirsch MA. Training with verbal instructional cues results in near-term improvement of gait in people with Parkinson disease. J Neurol Phys Ther. 2005;29(1):2-8.
34. Lim I, Van WE, Jones D, Rochester L, Nieuwboer A, Willems AM, et al. Does cueing training improve physical activity in patients with Parkinson’s disease? Neurorehabil Neural Repair. 2010;24(5):469-77.
35. Marjama-Lyons J, Smith L, Mylar B, Nelson J, Holliday G, Seracino D. Tai Chi and reduced rate of falling in Parkinson’s disease: A single-blinded pilot study. Mov Disord. 2002;17 (Suppl 5):190.
36. Modugno N, Iaconelli S, Fiorlli M, Lena F, Kusch I, Mirabella G. Active theater as a complementary therapy for Parkinson’s disease rehabilitation: a pilot study. Sci World J. 2010;10:2301-13.
37. Muller V, Mohr B, Rosin R, Pulvermuller F, Muller F, Birbaumer N. Short-term effects of behavioral treatment on movement initiation and postural control in Parkinson’s disease: a controlled clinical study. Mov Disord. 1997;12(3):306-14.
38. Pacchetti C, Mancini F, Aglieri R, Fundaro C, Martignoni E, Nappi G. Active music therapy in Parkinson’s disease: an integrative method for motor and emotional rehabilitation. Psychosom Med. 2000;62(3):386-93.
39. Palmer SS, Mortimer JA, Webster DD, Bistevins R, Dickinson GL. Exercise therapy for Parkinson’s disease. Arch Phys Med Rehabil. 1986;67(10):741-5.
40. Patti F, Reggio A, Nicoletti F, Sellaroli T, Deinite G, Nicoletti F. Effects of rehabilitation therapy on Parkinsons’ disability and functional independence. J Neurol Rehabil. 1996;14(4):223-31.
41. Purchas MA, MacMahon DG. The effects of Tai Chi training on general wellbeing and motor performance in patients with Parkinson’s disease (PD): A pilot study. Mov Disord. 2007;22 (Suppl 16):260.
42. Reuter I, Mehnert S, Oechsner M, Engelhardt M. Cognitive rehabilitation in Parkinson’s disease using neuropsychological training, transfer training and sports therapy. In: Dushanova J, editor. Diagnostics and Rehabilitation of Parkinson’s Disease: InTech; 2011. p. 257-86.
43. Schilling BK, LeDoux MS, Pfeiffer RF, Karlage RE, Weiss LW, Falvo MJ. Effects of lower-body resistance training in persons with Parkinson’s disease. Mov Disord. 2008;23 (Supl 1):639.
44. Shiba YS, Obuchi S, Toshima H, Yamakita H, editors. Comparison between visual and auditory stimulation in gait training of patients with idiopathic Parkinson’s disease. World Congress of Physical Therapy Conference; 1999.
45. Stallibrass C, Sissons P, Chalmers C. Randomized controlled trial of the Alexander technique for idiopathic Parkinson’s disease. Clin Rehabil. 2002;16(7):695-708.
46. Tamir R, Dickstein R, Huberman M. Integration of motor imagery and physical practice in group treatment applied to subjects with Parkinson’s disease. Neurorehabil Neural Repair. 2007;21(1):68-75.
47. Tanaka K, Quadros AC, Jr., Santos RF, Stella F, Gobbi LT, Gobbi S. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn. 2009;69(2):435-41.
48. Tickle-DegnenL,EllisT,Saint-HilaireMH,ThomasCA,WagenaarRC.Self-management rehabilitation and health-related quality of life in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2010;25(2):194-204.
49. Troche MS, Okun MS, Rosenbek JC, Musson N, Fernandez HH, Rodriguez R, et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: a randomized trial. Neurology. 2010;75(21):1912-9.
50. Gerpen JA van, Saucier M, Matthews M. Attenuating gait freezing and stride reductionin Parkinson patients with an attachable, adjustable laser (the mMobilaser TM): a pilot trial. Parkinsonism Relat Disord. 2010;16 (Suppl 1):S85.
51. Wade DT, Gage H, Owen C, Trend P, Grossmith C, Kaye J. Multidisciplinary rehabilitation for people with Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2003;74(2):158-62.
52. Wells MR, Giantinoto S, D’Agate D, Areman RD, Fazzini EA, Dowling D, et al. Standard osteopathic manipulative treatment acutely improves gait performance in patients with Parkinson’s disease. J Am Osteopath Assoc. 1999;99(2):92-8.
53. White DK, Wagenaar RC, Ellis TD, Tickle-Degnen L. Changes in walking activity and endurance following rehabilitation for people with Parkinson disease. Arch Phys Med Rehabil. 2009;90(1):43-50.
54. Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther. 2011;91(6):862-74.
55. Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, Close JC, et al. The effects of an exercise program on fall risk factors in people with Parkinson’s disease: a randomized controlled trial. Mov Disord. 2010;25(9):1217-25.
56. Almeida QJ, Bhatt H. A Manipulation of Visual Feedback during Gait Training in Parkinson’s Disease. Parkinsons Dis. 2012;2012:508720.
57. Arias P, Chouza M, Vivas J, Cudeiro J. Effect of whole body vibration in Parkinson’s disease: a controlled study. Mov Disord. 2009;24(6):891-8.
58. Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home-based exercise programme to reduce the risk of falling among people with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(7):678-84.
59. Braun S, Beurskens A, Kleynen M, Schols J, Wade D. Rehabilitation with mental practice has similar effects on mobility as rehabilitation with relaxation in people with Parkinson’s disease: a multicentre randomised trial. J Physiother. 2011;57(1):27-34.
60. Bridgewater KJ, Sharpe M. Trunk muscle training and early Parkinson’s disease. Physiother Th Pract. 1997;13(2):139-53.
61. Caglar AT, Gurses HN, Mutluay FK, Kiziltan G. Effects of home exercises on motor performance in patients with Parkinson’s disease. Clin Rehabil. 2005;19(8):870-7.
62. Cakit BD, Saracoglu M, Genc H, Erdem HR, Inan L. The effects of incremental speed-dependent treadmill training on postural instability and fear of falling in Parkinson’s disease. Clin Rehabil. 2007;21(8):698-705.
63. Canning CG, Allen NE, Dean CM, Goh L, Fung VS. Home-based treadmill training for individuals with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil. 2012;26(9):817-26.
64. Chandler C, Plant R. A targeted physiotherapy service for people with Parkinson’s disease from diagnosis to end stage: a pilot study. In: Percival R, Hobson P, editors. Parkinson’s disease: Studies in psychological and social care. Leicester: BPS Books; 1999. p. 256-69.
65. Christofoletti G, Beinotti F, Borges G, Damasceno BP. Physical therapy improves the balance of patients with parkinson’s disease: a randomized controlled trial. Parkinsonism Relat Disord. 2010;16 (Suppl 1):S58.
66. Comella CL, Stebbins GT, Brown-Toms N, Goetz CG. Physical therapy and Parkinson’s disease: a controlled clinical trial. Neurology. 1994;44(3 Pt 1):376-8.67. Craig LH, Svircev A, Haber M, Juncos JL. Controlled pilot study of the effects of neuromuscular therapy in patients with Parkinson’s disease. Mov Disord. 2006;21(12):2127-33.
68. Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, Thomas MG. Exercise and Parkinson’s: benefits for cognition and quality of life. Acta NeurolScand. 2011;123(1):13-9.
69. Bruin N de, Doan JB, Turnbull G, Suchowersky O, Bonfield S, Hu B, et al. Walking with music is a safe and viable tool for gait training in Parkinson’s disease: the effect of a 13-week feasibility study on single and dual task walking. Parkinsons Dis. 2010;2010:483530.
70. Iansek R. Interdisciplinary rehabilitation in Parkinson’s disease. In: Stern GM, ed., editors. Advances in Neurology Parkinson’s disease. 80 ed. Philidelphia: Lippincot Williams & Wilkins; 1999. p. 555-9.
71. Dereli EE, Yaliman A. Comparison of the effects of a physiotherapistsupervised exercise programme and a self-supervised exercise programme on quality of life in patients with Parkinson’s disease. Clin Rehabil. 2010;24(4):352-62.
72. Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov Disord. 2006;21(9):1444-52.
73. Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves Quality Of Life in persons with Parkinson’s disease: a preliminary study. Parkinsonism Relat Disord. 2009;15(10):752-7.
74. Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132-43.
75. Ebersbach G, Edler D, Kaufhold O, Wissel J. Whole body vibration versus conventional physiotherapy to improve balance and gait in Parkinson’s disease. Arch Phys Med Rehabil. 2008;89(3):399-403.
76. Ebersbach G, Ebersbach A, Edler D, Kaufhold O, Kusch M, Kupsch A, et al. Comparing exercise in Parkinson’s disease--the Berlin LSVT(R)BIG study. Mov Disord. 2010;25(12):1902-8.
77. Ellis T, Goede CJ de, Feldman RG, Wolters EC, Kwakkel G, Wagenaar RC. Efficacy of a physical therapy program in patients with Parkinson’s disease: A randomized controlled trial. Arch Phys Med Rehabil. 2005;86(4):626-32.
78. Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil. 2008;89(7):1221-9.
79. Frazzitta G, Maestri R, Uccellini D, Bertotti G, Abelli P. Rehabilitation treatment of gait in patients with Parkinson’s disease with freezing: A comparison between two physical therapy protocols using visual and auditory cues with or without treadmill training. Mov Disord. 2009. Jun 15;24(8):1139-43.
80. Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson’s disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry. 2011;82(11):1232-8.
81. Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. 2009;41(6):475-81.
82. Hackney ME, Earhart GM. Tai Chi improves balance and mobility in people with Parkinson disease. Gait Posture. 2008;28(3):456-60.
83. Hackney ME, Earhart GM. Effects of dance on gait and balance in Parkinson’s disease: a comparison of partnered and nonpartnered dance movement. Neurorehabil Neural Repair. 2010;24(4):384-92.
84. Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neurol Phys Ther. 2007;31(4):173-9.
85. Hass CJ, Buckley TA, Pitsikoulis C, Barthelemy EJ. Progressive resistance training improves gait initiation in individuals with Parkinson’s disease. Gait Posture. 2012;35(4):669-73.
86. Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch Phys Med Rehabil. 2003;84(8):1109-17.
87. Kadivar Z, Corcos DM, Foto J, Hondzinski JM. Effect of step training and rhythmic auditory stimulation on functional performance in Parkinson patients. Neurorehabil Neural Repair. 2011;25(7):626-35.
88. Kamsma YPT, Brouwer WH, Lakke JPWF. Training of compensatory strategies for impaired gross motor skills in patients with Parkinson’s disease. Physiother Th Pract. 1995;11:209-29.
89. Keus SH, Bloem BR, Hilten JJ van, Ashburn A, Munneke M. Effectiveness of physiotherapy in Parkinson’s disease: the feasibility of a randomised controlled trial. Parkinsonism Relat Disord. 2007;13(2):115-21.
90. Klassen L, Dal Bello-Haas V, Sheppard M, Metcalfe A. Evaluating the benefits of group exercise and group exercise and education programs for individuals with Parkinson’s disease. Physiotherapy. 2007;93 (Suppl. 1):S91.
91. Kurtais Y, Kutlay S, Tur BS, Gok H, Akbostanci C. Does treadmill training improve lower-extremity tasks in Parkinson disease? A randomized controlled trial. Clin J Sport Med. 2008;18(3):289-91.
92. Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366(6):511-9.
93. Lun V, Pullan N, Labelle N, Adams C, Suchowersky O. Comparison of the effects of a self-supervised home exercise program with a physiotherapist-supervised exercise program on the motor symptoms of Parkinson’s disease. Mov Disord. 2005;20(8):971-5.
94. Mak MK, Hui-Chan CW. Cued task-specific training is better than exercise in improving sit-to-stand in patients with Parkinson’s disease: A randomized controlled trial. Mov Disord. 2008;23(4):501-9.
95. Marchese R, Diverio M, Zucchi F, Lentino C, Abbruzzese G. The role of sensory cues in the rehabilitation of parkinsonian patients: a comparison of two physical therapy protocols. Mov Disord. 2000;15(5):879-83.
96. Meek C, Sackley CM, C.E. C, Soundy AA, Winward C, Esser P, et al. Longterm individual fitness enablement (LIFE) for Parkinson’s disease:a feasibility study. Mov Disord. 2010;25 (Suppl 3):S713.
97. Miyai I, Fujimoto Y, Ueda Y, Yamamoto H, Nozaki S, Saito T, et al. Treadmill training with body weight support: its effect on Parkinson’s disease. Arch Phys Med Rehabil. 2000;81(7):849-52.
98. Miyai I, Fujimoto Y, Yamamoto H, Ueda Y, Saito T, Nozaki S, et al. Longterm effect of body weight-supported treadmill training in Parkinson’s disease: a randomized controlled trial. Arch Phys Med Rehabil. 2002;83(10):1370-3.
99. Mohr B, Muller V, Mattes R, Rosin R, Federmann B, Strehl U, et al. Behavioral treatment of Parkinson’s disease leads to improvement of motor skills and tremor reduction. Behav Ther. 1996;27:235-55.
100. Morris ME, Iansek R, Kirkwood B. A randomized controlled trial of movement strategies compared with exercise for people with Parkinson’s disease. Mov Disord. 2009;24(1):64-71.
101. Nieuwboer A, Weerdt W de, Dom R, Truyen M, Janssens L, Kamsma Y. The effect of a home physiotherapy program for persons with Parkinson’s disease. J Rehabil Med. 2001;33(6):266-72.
102. Nieuwboer A, Kwakkel G, Rochester L, Jones D, Wegen E van, Willems AM, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78(2):134-40.
103. Pelosin E, Avanzino L, Bove M, Stramesi P, Nieuwboer A, Abbruzzese G. Action observation improves freezing of gait in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2010;24(8):746-52.
104. Pohl M, Rockstroh G, Ruckriem S, Mrass G, Mehrholz J. Immediate effects of speed-dependent treadmill training on gait parameters in early Parkinson’s disease. Arch Phys Med Rehabil. 2003;84(12):1760-6.
105. Pompeu JE, Mendes FA, Silva KG, Lobo AM, Oliveira TP, Zomignani AP, et al. Effect of Nintendo Wii-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy. 2012;98(3):196-204.
106. Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson’s disease. Neuro Rehabil. 2005;20(3):183-90.
107. Reuter I, Mehnert S, Leone P, Kaps M, Oechsner M, Engelhardt M. Effects of a flexibility and relaxation programme, walking, and nordic walking on Parkinson’s disease. J Aging Res. 2011;2011:232473.
108. Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair. 2009;23(6):600-8.
109. Sage MD, Almeida QJ. Symptom and gait changes after sensory attention focused exercise vs aerobic training in Parkinson’s disease. Mov Disord. 2009. Jun 15;24(8):1132-8.
110. Sage MD, Almeida QJ. A positive influence of vision on motor symptoms during sensory attention focused exercise for Parkinson’s disease. Mov Disord. 2010;25(1):64-9.
111. Schenkman M, Cutson TM, Kuchibhatla M, Chandler J, Pieper CF, Ray L, et al. Exercise to improve spinal flexibility and function for people with Parkinson’s disease: a randomized, controlled trial. J Am Geriatr Soc. 1998;46(10):1207-16.
112. Schenkman M, Hall DA, Baron AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for people in early or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther. 2012;92(11):1395-410.
113. Schilling BK, Pfeiffer RF, LeDoux MS, Karlage RE, Bloomer RJ, Falvo MJ. Effects of moderate-volume, high-load lower-body resistance training on strength and function in persons with Parkinson’s disease: a pilot study. Parkinsons Dis. 2010;2010:824734.
114. Schmitz-Hubsch T, Pyfer D, Kielwein K, Fimmers R, Klockgether T, Wullner U. Qigong exercise for the symptoms of Parkinson’s disease: a random ized, controlled pilot study. Mov Disord. 2006;21(4):543-8.
115. Shankar A, Bruin N de, Bonfield S, Derwent L, Eliasziw M, Hu B, et al. Benefit of music therapy in patients with Parkinson’s disease: a randomized controlled trial. Mov Disord. 2008;23((Suppl 1)):68.
116. Smania N, Corato E, Tinazzi M, Stanzani C, Fiaschi A, Girardi P, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabil Neural Repair. 2010;24(9):826-34.
117. Stack E, Roberts H, Ashburn A. The PIT: SToPP Trial-A Feasibility Randomised Controlled Trial of Home-Based Physiotherapy for People with Parkinson’s Disease Using Video-Based Measures to Preserve Assessor Blinding. Parkinsons Dis. 2012;2012:360231.
118. Stozek J, Rudzinska M, Longawa K, Szczudlik A. [The effect of the complex rehabilitation on posture and gait in Parkinson disease]. Neurol Neurochir Pol. 2003;37 Suppl 5:67-81.
119. Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, Brault JM. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov Disord. 1996;11(2):193-200.
120. Toole T, Hirsch MA, Forkink A, Lehman DA, Maitland CG. The effects of a balance and strength training program on equilibrium in Parkinsonism: A preliminary study. Neuro Rehabil. 2000;14(3):165-74.
121. Toole T, Maitland CG, Warren E, Hubmann MF, Panton L. The effects of loading and unloading treadmill walking on balance, gait, fall risk, and daily function in Parkinsonism. Neuro Rehabil. 2005;20(4):307-22.
122. Vivas J, Arias P, Cudeiro J. Aquatic therapy versus conventional landbased therapy for Parkinson’s disease: an open-label pilot study. Arch Phys Med Rehabil. 2011;92(8):1202-10.
123. Winward C, Sackley C, Meek C, Izadi H, Barker K, Wade D, et al. Weekly exercise does not improve fatigue levels in Parkinson’s disease. Mov Disord. 2012;27(1):143-6.
124. Yang YR, Lee YY, Cheng SJ, Wang RY. Downhill walking training in individuals with Parkinson’s disease: a randomized controlled trial. Am J Phys Med Rehabil. 2010;89(9):706-14.
125. Yousefi B, Tadibi V, Khoei AF, Montazeri A. Exercise therapy, quality of life, and activities of daily living in patients with Parkinson disease: a small scale quasi-randomised trial. Trials. 2009;10:67.
126. Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336(7652):1049-51.
127. Bloem BR, Laar T van, Keus SHJ, Beer H de, Poot E, Buskens E, et al. Multidisciplinary Guideline ‘Parkinson’s disease’ [Multidisciplinaire richtlijn ziekte van Parkinson]. Alphen aan den Rijn: Van Zuiden Communications; 2010.
128. Taylor KS, Cook JA, Counsell CE. Heterogeneity in male to female risk for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(8):905-6.
129. Lau LM de, Koudstaal PJ, Hofman A, Breteler MM. [Parkinson disease is more prevalent than people think. Research results]. Ned Tijdschr Geneeskd. 2009;153(3):63-8.
130. Campenhausen S von, Bornschein B, Wick R, Botzel K, Sampaio C, Poewe W, et al. Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharmacol. 2005;15(4):473-90.
131. Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):155-62.
132. Lindgren P, Campenhausen S von, Spottke E, Siebert U, Dodel R. Cost of Parkinson’s disease in Europe. Eur J Neurol. 2005;12 Suppl 1:68-73.
133. Findley LJ. The economic impact of Parkinson’s disease. Parkinsonism Relat Disord. 2007;13 Suppl:S8-S12.
134. Keranen T, Kaakkola S, Sotaniemi K, Laulumaa V, Haapaniemi T, Jolma T, et al. Economic burden and quality of life impairment increase with severity of PD. Parkinsonism Relat Disord. 2003;9(3):163-8.
135. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33-9.
136. Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368-76.
137. Aerts MB, Esselink RA, Post B, Warrenburg BP van de, Bloem BR. Improving the diagnostic accuracy in parkinsonism: a three-pronged approach. Pract Neurol. 2012;12(2):77-87.
138. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-4.
139. Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57(8):1497-9.
140. Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson’s disease in the community? J Neurol Neurosurg Psychiatry. 2002;73(5):529-34.
141. Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(Pt 4):861-70.
142. Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006;5(1):75-86.
143. Obeso JA, Rodriguez-Oroz MC, itez-Temino B, Blesa FJ, Guridi J, Marin C, et al. Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Disord. 2008;23 Suppl 3:S548-S59.
144. Braak H, Del TK. Cortico-basal ganglia-cortical circuitry in Parkinson’s disease reconsidered. Exp Neurol. 2008;212(1):226-9.
145. Maele-Fabry G van, Hoet P, Vilain F, Lison D. Occupational exposure to pesticides and Parkinson’s disease: a systematic review and meta-analysis of cohort studies. Environ Int. 2012;46:30-43.
146. Kiyohara C, Kusuhara S. Cigarette smoking and Parkinson’s disease: a meta-analysis. Fukuoka Igaku Zasshi. 2011;102(8):254-65.
147. Allam MF, Campbell MJ, Del Castillo AS, Fernandez-Crehuet Navajas R. Parkinson’s disease protects against smoking? Behav Neurol. 2004;15 (3-4):65-71.
148. Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27(8):947-57.
149. Crosiers D, Theuns J, Cras P, Van BC. Parkinson disease: insights in clinical, genetic and pathological features of monogenic disease subtypes. J Chem Neuroanat. 2011;42(2):131-41.
150. Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol. 1993;50(2):140-8.
151. Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism – a prospective study. CanJ Neurol Sci. 1991;18(3):275-8.
152. Stamey W, Davidson A, Jankovic J. Shoulder pain: a presenting symptom of Parkinson disease. J Clin Rheumatol. 2008;14(4):253-4.
153. Song J, Sigward S, Fisher B, Salem GJ. Altered Dynamic Postural Control during Step Turning in Persons with Early-Stage Parkinson’s Disease. Parkinsons Dis. 2012;2012:386962.
154. Ziemssen T, Reichmann H. Non-motor dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(6):323-32.
155. Poewe W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15 Suppl 1:14-20.
156. Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69(3):308-12.
157. Chaudhuri KR, Prieto-Jurcynska C, Naidu Y, Mitra T, Frades-Payo B, Tluk S, et al. The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord. 2010;25(6):704-9.
158. Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235-45.
159. Chaudhuri KR, Naidu Y. Early Parkinson’s disease and non-motor issues. J Neurol. 2008;255 Suppl 5:33-8.
160. Ray CK, Rojo JM, Schapira AH, Brooks DJ, Stocchi F, Odin P, et al. A proposal for a comprehensive grading of Parkinson’s disease severity combining motor and non-motor assessments: meeting an unmet need. PLoSOne. 2013;8(2):e57221.
161. Muslimovic D, Schmand B, Speelman JD, Haan RJ de. Course of cognitive decline in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc. 2007;13(6):920-32.
162. Dirnberger G, Frith CD, Jahanshahi M. Executive dysfunction in Parkinson’s disease is associated with altered pallidal-frontal processing. Neuroimage. 2005;25(2):588-99.
163. Manning KJ, Clarke C, Lorry A, Weintraub D, Wilkinson JR, Duda JE, et al. Medication management and neuropsychological performance in Parkinson’s disease. Clin Neuropsychol. 2012;26(1):45-58.
164. Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-9.
165. Ha AD, Jankovic J. Pain in Parkinson’s disease. Mov Disord. 2012;27(4):485-91.
166. Leentjens AF, Dujardin K, Marsh L, Richard IH, Starkstein SE, Martinez-Martin P. Anxiety rating scales in Parkinson’s disease: a validation study of the Hamilton anxiety rating scale, the Beck anxiety inventory, and the hospital anxiety and depression scale. Mov Disord. 2011;26(3):407-15.
167. Santangelo G, Trojano L, Barone P, Errico D, Grossi D, Vitale C. Apathy in Parkinson’s disease: Diagnosis, neuropsychological correlates, pathophysiology and treatment. Behav Neurol. 2013. Jan 1;27(4):501-13.
168. Sturkenboom IHWM, Thijssen MCE, Gons-van de Elsacker JJ, Jansen IJH, Maasdam A, Schulten M, et al. Ergotherapie bij de ziekte van Parkinson, een richtlijn van Ergotherapie Nederland. Utrecht/Den Haag: Ergotherapie Nederland/Uitgeverij Lemma; 2008.
169. Visser M, Rooden SM van, Verbaan D, Marinus J, Stiggelbout AM, Hilten JJ van. A comprehensive model of health-related quality of life in Parkinson’s disease. J Neurol. 2008;255(10):1580-7.
170. Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord. 2008;23(10):1428-34.
171. Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Caregiver-burden in Parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12(1):35-41.
172. Hariz GM, Forsgren L. Activities of daily living and quality of life in persons with newly diagnosed Parkinson’s disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol Scand. 2011;123(1):20-7.
173. Schenkman M, Ellis T, Christiansen C, Baron AE, Tickle-Degnen L, Hall DA, et al. Profile of functional limitations and task performance among people with earlyand middle-stage Parkinson disease. Phys Ther. 2011;91(9):1339-54.
174. Shulman LM, Gruber-Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS, et al. The evolution of disability in Parkinson disease. Mov Disord. 2008;23(6):790-6.
175. Evans JR, Mason SL, Williams-Gray CH, Foltynie T, Brayne C, Robbins TW, et al. The natural history of treated Parkinson’s disease in an incident, community based cohort. J Neurol Neurosurg Psychiatry. 2011;82(10):1112-8.
176. Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020-8.
177. NICE. Parkinson’s disease. Diagnosis and management in primary and secondary care (NICE Clinical Guideline 35). London, UK: National collaborating centre for chronic conditions; 2006 2006.
178. Sato K, Hatano T, Yamashiro K, Kagohashi M, Nishioka K, Izawa N, et al. Prognosis of Parkinson’s disease: time to stage III, IV, V, and to motor fluctuations. Mov Disord. 2006;21(9):1384-95.
179. Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529-34.
180. Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005;76(3):343-8.
181. Reijnders JS, Ehrt U, Lousberg R, Aarsland D, Leentjens AF. The association between motor subtypes and psychopathology in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(5):379-82.
182. Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132(Pt 11):2947-57.
183. Contreras A, Grandas F. Risk factors for freezing of gait in Parkinson’s disease. J Neurol Sci. 2012.
184. Burn DJ, Landau S, Hindle JV, Samuel M, Wilson KC, Hurt CS, et al. Parkinson’s disease motor subtypes and mood. Mov Disord. 2012;27(3):379-86.
185. Berg WD van de, Hepp DH, Dijkstra AA, Rozemuller JA, Berendse HW, Foncke E. Patterns of alpha-synuclein pathology in incidental cases and clinical subtypes of Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S28-S30.
186. Roos RA, Jongen JC, Velde EA van der. Clinical course of patients with idiopathic Parkinson’s disease. Mov Disord. 1996;11(3):236-42.
187. Starkstein SE, Petracca G, Chemerinski E, Teson A, Sabe L, Merello M, et al. Depression in classic versus akinetic-rigid Parkinson’s disease. Mov Disord. 1998;13(1):29-33.
188. Leibson CL, Maraganore DM, Bower JH, Ransom JE, O’Brien PC, Rocca WA. Comorbid conditions associated with Parkinson’s disease: a population-based study. Mov Disord. 2006;21(4):446-55.
189. Jones JD, Malaty I, Price CC, Okun MS, Bowers D. Health comorbidities and cognition in 1948 patients with idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(10):1073-8.
190. Martignoni E, Godi L, Citterio A, Zangaglia R, Riboldazzi G, Calandrella D, et al. Comorbid disorders and hospitalisation in Parkinson’s disease: a prospective study. Neurological Sciences. 2004;25(2):66-71.
191. Pressley JC, Louis ED, Tang MX, Cote L, Cohen PD, Glied S, et al. The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology. 2003;60(1):87-93.
192. Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380(9836):7-9.
193. Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288-94.
194. Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridinelesioned mouse basal ganglia. J Neurosci Res. 2004;77(3):378-90.
195. Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27(20):5291-300.
196. Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, et al. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res. 2010;1310:200-7.
197. Aerts MB, Eijk EM van der, Kramers K, Bloem BR. [Insufficient medication compliance in Parkinson’s disease. Ned Tijdschr Geneeskd. 2011;155:A3031.
198. Daley DJ, Myint PK, Gray RJ, Deane KH. Systematic review on factors associated with medication non-adherence in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(10):1053-61.
199. Grosset KA, Bone I, Grosset DG. Suboptimal medication adherence in Parkinson’s disease. Mov Disord. 2005;20(11):1502-7.
200. Katzenschlager R, Head J, Schrag A, Ben-Shlomo Y, Evans A, Lees AJ. Fourteen-year final report of the randomized PDRG-UK trial comparing three initial treatments in PD. Neurology. 2008;71(7):474-80.
201. Cereda E, Barichella M, Pedrolli C, Pezzoli G. Low-protein and protein-redistribution diets for Parkinson’s disease patients with motor fluctuations: a systematic review. Mov Disord. 2010;25(13):2021-34.
202. Robertson DR, Higginson I, Macklin BS, Renwick AG, Waller DG, George CF. The influence of protein containing meals on the pharmacokinetics of levodopa in healthy volunteers. Br J Clin Pharmacol. 1991;31(4):413-7.
203. Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10(8):734-44.
204. Volkmann J. Update on surgery for Parkinson’s disease. Curr Opin Neurol. 2007;20(4):465-9.
205. Okun MS, Foote KD. Parkinson’s disease DBS: what, when, who and why? The time has come to tailor DBS targets. Expert Rev Neurother. 2010;10(12):1847-57.
206. Meek CE. Improving the clinical effectiveness of physiotherapy in Parkinson’s disease. Birmingham: University of Birmingham; 2011.
207. Keus SH, Bloem BR, Hendriks EJ, Bredero-Cohen AB, Munneke M. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord. 2007;22(4):451-60.
208. Morris ME. Movement disorders in people with Parkinson disease: a model for physical therapy. Phys Ther. 2000;80(6):578-97.
209. Fertl E, Doppelbauer A, Auff E. Physical activity and sports in patients suffering from Parkinson’s disease in comparison with healthy seniors. J Neural Transm Park Dis Dement Sect. 1993;5(2):157-61.
210. Nimwegen M van, Speelman AD, Hofman-van Rossum EJ, Overeem S, Deeg DJ, Borm GF, et al. Physical inactivity in Parkinson’s disease. J Neurol. 2011;258(12):2214-21.
211. Ellis T, Cavanaugh JT, Earhart GM, Ford MP, Foreman KB, Fredman L, et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther. 2011;91(12):1838-48.
212. Nilsson MH, Drake AM, Hagell P. Assessment of fall-related self-efficacy and activity avoidance in people with Parkinson’s disease. BMC Geriatr. 2010;10:78.
213. Inkster LM, Eng JJ, MacIntyre DL, Stoessl AJ. Leg muscle strength is reduced in Parkinson’s disease and relates to the ability to rise from a chair. Mov Disord. 2003;18(2):157-62.
214. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67(1):28-40.
215. Allen NE, Sherrington C, Canning CG, Fung VS. Reduced muscle power is associated with slower walking velocity and falls in people with Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(4):261-4.
216. Paul SS, Sherrington C, Fung VS, Canning CG. Motor and Cognitive Impairments in Parkinson Disease: Relationships With Specific Balance and Mobility Tasks. Neurorehabil Neural Repair. 2012.
217. Paul SS, Canning CG, Sherrington C, Fung VS. Reduced muscle strength is the major determinant of reduced leg muscle power in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(8):974-7.
218. Schilling BK, Karlage RE, LeDoux MS, Pfeiffer RF, Weiss LW, Falvo MJ. Impaired leg extensor strength in individuals with Parkinson disease and relatedness to functional mobility. Parkinsonism Relat Disord. 2009;15(10):776-80.
219. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219-29.
220. Vitale C, Santangelo G, Verde F, Amboni M, Sorrentino G, Grossi D, et al. Exercise dependence induced by pramipexole in Parkinson’s Disease-a case report. Mov Disord. 2010;25(16):2893-4.
221. Abosch A, Gupte A, Eberly LE, Tuite PJ, Nance M, Grant JE. Impulsive behavior and associated clinical variables in Parkinson’s disease. Psychosomatics. 2011;52(1):41-7.
222. Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110 ( Pt 2):361-79.
223. Morris ME, Iansek R. Characteristics of motor disturbance in Parkinson’s disease and strategies for movement rehabilitation. Human Mov Sci. 1996;15:649-69.
224. Kamsma Y. Functional reorganisation of basic motor actions in Parkinson’s disease [thesis]. Groningen: Rijksuniversiteit Groningen; 2002.
225. Mak MK, Yang F, Pai YC. Limb collapse, rather than instability, causes failure in sit-to-stand performance among patients with parkinson disease. Phys Ther. 2011;91(3):381-91.
226. Bertram CP, Lemay M, Stelmach GE. The effect of Parkinson’s disease on the control of multi-segmental coordination. Brain Cogn. 2005;57(1):16-20.
227. Fellows SJ, Noth J, Schwarz M. Precision grip and Parkinson’s disease. Brain. 1998;121 (Pt 9):1771-84.
228. Fellows SJ, Noth J. Grip force abnormalities in de novo Parkinson’s disease. Mov Disord. 2004;19(5):560-5.
229. Baumann CR. Epidemiology, diagnosis and differential diagnosis in Parkinson’s disease tremor. Parkinsonism Relat Disord. 2012;18 Suppl 1:S90-S2.
230. Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56(12):1712-21.
231. Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol. 2001;248(11):950-8.
232. Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord. 2007;22(13):1892-900.
233. Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72(6):721-5.
234. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837-44.
235. Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, et al. Proprioception and motor control in Parkinson’s disease. J Mot Behav. 2009;41(6):543-52.
236. Schenkman M, Morey M, Kuchibhatla M. Spinal flexibility and balance control among community-dwelling adults with and without Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2000;55(8):M441-M5.
237. Wenning GK, Ebersbach G, Verny M, Chaudhuri KR, Jellinger K, McKee A, et al. Progression of falls in postmortem-confirmed parkinsonian disorders. Mov Disord. 1999;14(6):947-50.
238. Worringham CJ, Stelmach GE. Practice effects on the preprogramming of discrete movements in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1990;53(8):702-4.
239. Wielinski CL, Erickson-Davis C, Wichmann R, Walde-Douglas M, Parashos SA. Falls and injuries resulting from falls among patients with Parkinson’s disease and other parkinsonian syndromes. Mov Disord. 2005;20(4):410-5.
240. Chen YY, Cheng PY, Wu SL, Lai CH. Parkinson’s disease and risk of hip fracture: an 8-year follow-up study in Taiwan. Parkinsonism Relat Disord. 2012;18(5):506-9.
241. Bhattacharya RK, Dubinsky RM, Lai SM, Dubinsky H. Is there an increased risk of hip fracture in Parkinson’s disease? A nationwide inpatient sample. Mov Disord. 2012;27(11):1440-3.
242. Sato Y, Manabe S, Kuno H, Oizumi K. Amelioration of osteopenia and hypovitaminosis D by 1alpha-hydroxyvitamin D3 in elderly patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;66(1):64-8.
243. Jonsson B, Sernbo I, Johnell O. Rehabilitation of hip fracture patients with Parkinson’s Disease. Scand J Rehabil Med. 1995;27(4):227-30.
244. Idjadi JA, Aharonoff GB, Su H, Richmond J, Egol KA, Zuckerman JD, et al. Hip fracture outcomes in patients with Parkinson’s disease. Am J Orthop. 2005;34(7):341-6.
245. Ashburn A, Stack E, Pickering RM, Ward CD. A community-dwelling sample of people with Parkinson’s disease: characteristics of fallers and non-fallers. Age Ageing. 2001;30(1):47-52.
246. Carpenter MG, Allum JH, Honegger F, Adkin AL, Bloem BR. Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(9):1245-54.
247. Gray P, Hildebrand K. Fall risk factors in Parkinson’s disease. J Neurosci Nurs. 2000;32(4):222-8.
248. Bloem BR, Beckley DJ, Dijk JG van, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson’s disease. Mov Disord. 1996;11(5):509-21.
249. Bloem BR, Beckley DJ, Dijk JG van. Are automatic postural responses in patients with Parkinson’s disease abnormal due to their stooped posture? ExpBrain Res. 1999;124(4):481-8.
250. Ashburn A, Stack E, Pickering RM, Ward CD. Predicting fallers in a community-based sample of people with Parkinson’s disease. Gerontology. 2001;47(5):277-81.
251. Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson’s disease. Mov Disord. 2003;18(5):496-502.
252. Franchignoni F, Martignoni E, Ferriero G, Pasetti C. Balance and fear of falling in Parkinson’s disease. Parkinsonism Relat Disord. 2005;11(7):427-33.
253. Mak MK, Pang MY. Fear of falling is independently associated with recurrent falls in patients with Parkinson’s disease: a 1-year prospective study. J Neurol. 2009.
254. Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. On the nature of fear of falling in Parkinson’s disease. Behav Neurol. 2011;24(3):219-28.
255. Mak MK, Pang MY. Balance confidence and functional mobility are independently associated with falls in people with Parkinson’s disease. J Neurol. 2009;256(5):742-9.
256. Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson’s disease: frequency, impact and identifying factors. J Neurol. 2005;252(11):1310-5.
257. Koerts J, Van BM, Tucha O, Leenders KL, Brouwer WH. Executive functioning in daily life in Parkinson’s disease: initiative, planning and multitask performance. PLoSOne. 2011;6(12):e29254.
258. Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis. 2012;2012:918719.
259. Allcock LM, Rowan EN, Steen IN, Wesnes K, Kenny RA, Burn DJ. Impaired attention predicts falling in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(2):110-5.
260. Bloem BR, Grimbergen YA, Dijk JG van, Munneke M. The ‘posture second’ strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci. 2006;248(1-2):196-204.
261. Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16(1):53-8.
262. Marchese R, Bove M, Abbruzzese G. Effect of cognitive and motor tasks on postural stability in Parkinson’s disease: A posturographic study. Mov Disord. 2003;18(6):652-8.
263. Hausdorff JM. Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19(026113).
264. Mak MK. Reduced step length, not step length variability is central to gait hypokinesia in people with Parkinson’s disease. Clin Neurol Neurosurg. 2012.
265. Ehgoetz Martens KA, Pieruccini-Faria F, Almeida QJ. Could sensory mechanisms be a core factor that underlies freezing of gait in Parkinson’s disease? PLoSOne. 2013;8(5):e62602.
266. Hass CJ, Malczak P, Nocera J, Stegemoller EL, Wagle SA, Malaty I, et al. Quantitative normative gait data in a large cohort of ambulatory persons with Parkinson’s disease. PLoSOne. 2012;7(8):e42337.
267. LaPlante J, Kaeser TP, editors. A history of pedestrian signal walking speed assumptions. Seattle, Washington: 3rd Urban Street Symposium; 2007.
268. Nemanich ST, Duncan RP, Dibble LE, Cavanaugh JT, Ellis TD, Ford MP, et al. Predictors of gait speeds and the relationship of gait speeds to falls in men and women with Parkinson disease. Parkinsons Dis. 2013;2013:141720.
269. Paul SS, Canning CG, Sherrington C, Lord SR, Close JC, Fung VS. Three simple clinical tests to accurately predict falls in people with Parkinson’s disease. Mov Disord. 2013;28(5):655-62.
270. Tan D, Danoudis M, McGinley J, Morris ME. Relationships between motor aspects of gait impairments and activity limitations in people with Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2012;18(2):117-24.
271. Matinolli M, Korpelainen JT, Sotaniemi KA, Myllyla VV, Korpelainen R. Recurrent falls and mortality in Parkinson’s disease: a prospective two-year follow-up study. Acta Neurol Scand. 2011;123(3):193-200.
272. Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non-freezer: Clinical assessment of freezing of gait. Parkinsonism Relat Disord. 2012;18(2):149-54.
273. Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;23 Suppl 2:S423-S5.
274. Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol. 2003;10(4):391-8.
275. Giladi N. Freezing of gait. Clinical overview. Adv Neurol. 2001;87:191-7.
276. Tan DM, McGinley JL, Danoudis ME, Iansek R, Morris ME. Freezing of gait and activity limitations in people with Parkinson’s disease. Arch Phys Med Rehabil. 2011;92(7):1159-65.
277. Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov Disord. 2004;19(8):871-84.
278. Morris ME. Locomotor training in people with Parkinson disease. Phys Ther. 2006;86(10):1426-35.
279. Snijders AH, Warrenburg BP van de, Giladi N, Bloem BR. Neurological gait disorders in elderly people: clinical approach and classification. Lancet Neurol. 2007;6(1):63-74.
280. Vercruysse S, Devos H, Munks L, Spildooren J, Vandenbossche J, Vandenberghe W, et al. Explaining freezing of gait in Parkinson’s disease: motor and cognitive determinants. Mov Disord. 2012;27(13):1644-51.
281. Del SF, Albanese A. Clinical management of pain and fatigue in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S233-S6.
282. Ford B. Pain in Parkinson’s disease. Clin Neurosci. 1998;5(2):63-72.
283. Fil A, Cano-de-la-Cuerda R, Munoz-Hellin E, Vela L, Ramiro-Gonzalez M, Fernandez-de-Las-Penas C. Pain in Parkinson disease: a review of the literature. Parkinsonism Relat Disord. 2013;19(3):285-94.
284. Gerdelat-Mas A, Simonetta-Moreau M, Thalamas C, Ory-Magne F, Slaoui T, Rascol O, et al. Levodopa raises objective pain threshold in Parkinson’s disease: a RIII reflex study. J Neurol Neurosurg Psychiatry. 2007;78(10):1140-2.
285. Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26(42):10789-95.
286. Scherder E, Wolters E, Polman C, Sergeant J, Swaab D. Pain in Parkinson’s disease and multiple sclerosis: its relation to the medial and lateral pain systems. Neurosci Biobehav Rev. 2005;29(7):1047-56.
287. Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153(6):1144-7.
288. Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30(1):77-94.
289. Truchon M, Cote D, Fillion L, Arsenault B, Dionne C. Low-back-pain related disability: an integration of psychological risk factors into the stress process model. Pain. 2008;137(3):564-73.
290. Ford B. Pain in Parkinson’s disease. Mov Disord. 2010;25 Suppl 1:S98-103.
291. Mehanna R, Jankovic J. Respiratory problems in neurologic movement disorders. Parkinsonism Relat Disord. 2010;16(10):628-38.
292. Shill H, Stacy M. Respiratory complications of Parkinson’s disease. Semin Respir Crit Care Med. 2002;23(3):261-5.
293. De Pandis MF, Starace A, Stefanelli F, Marruzzo P, Meoli I, De Simone G, et al. Modification of respiratory function parameters in patients with severe Parkinson’s disease. Neurol Sci. 2002;23 Suppl 2:S69-70.
294. Gerlach OH, Winogrodzka A, Weber WE. Clinical problems in the hospitalized Parkinson’s disease patient: systematic review. Mov Disord. 2011;26(2):197-208.
295. Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8(4):CR241-6.
296. Fall PA, Saleh A, Fredrickson M, Olsson JE, Granerus AK. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease: a 9-year follow-up. Mov Disord. 2003;18(11):1312-6.
297. Pennington S, Snell K, Lee M, Walker R. The cause of death in idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(7):434-7.
298. Kalf JG, Swart BJ de, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311-5.
299. Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135(5):1301-8.
300. Noradina AT, Karim NA, Hamidon BB, Norlinah I, Raymond AA. Sleep-disordered breathing in patients with Parkinson’s disease. Singapore Med J. 2010;51(1):60-4.
301. Silverman EP, Sapienza CM, Saleem A, Carmichael C, Davenport PW, Hoffman-Ruddy B, et al. Tutorial on maximum inspiratory and expiratory mouth pressures in individuals with idiopathic Parkinson disease (IPD) and the preliminary results of an expiratory muscle strength training program. Neuro Rehabil. 2006;21(1):71-9.
302. Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulm Pharmacol Ther. 2002;15(3):221-5.
303. Fontana GA, Lavorini F. Cough motor mechanisms. Respir Physiol Neurobiol. 2006;152(3):266-81.304. Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23(3):297-301.
305. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2-4.
306. Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20(6):64-78.
307. Wagner EH. Academia, chronic care, and the future of primary care. J Gen Intern Med. 2010;25 Suppl 4:S636-8.
308. Bengoa R, Kawar R, Key P, Leatherman S, Massoud R, Saturno P. Quality of care: a process for making strategic choices in health systems. Beschikbaar via: wwwwhoint/management/quality/assurance/QualityCare_BDefpdf. 2006.
309. Medicine Io. Crossing the quality chasm. A new health system for the 21st century. Beschikbaar via: http://booksnapedu/html/quality_chasm/ reportbriefpdf. 2001.
310. Grosset KA, Grosset DG. Patient-perceived involvement and satisfaction in Parkinson’s disease: effect on therapy decisions and quality of life. Mov Disord. 2005;20(5):616-9.
311. Nisenzon AN, Robinson ME, Bowers D, Banou E, Malaty I, Okun MS. Measurement of patient-centered outcomes in Parkinson’s disease: what do patients really want from their treatment? Parkinsonism Relat Disord. 2011;17(2):89-94.
312. Eijk M van der, Faber MJ, Ummels I, Aarts JW, Munneke M, Bloem BR. Patient-centeredness in PD care: development and validation of a patient experience questionnaire. Parkinsonism Relat Disord. 2012;18(9):1011-6.
313. Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48(2):177-87.
314. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-75.
315. Eijk M van der, Nijhuis FA, Faber MJ, Bloem BR. Moving from physician-centered care towards patient-centered care for Parkinson’s disease patients. Parkinsonism Relat Disord. 2013;19(11):923-7.
316. (ZonMw) MaHRCoTN. Executive Summary to the National Action Programme Self-management 2008-2012: knowledge, results and future [in Dutch]. Revalidatie Magazine. 2013;19(3):8-16.
317. Nijkrake MJ, Keus SH, Quist-Anholts GW, Overeem S, De Roode MH, Lindeboom R, et al. Evaluation of a Patient-Specific Index as an outcome measure for physiotherapy in Parkinson’s disease. Eur J Phys Rehabil Med. 2009;45(4):507-12.
318. Shine JM, Moore ST, Bolitho SJ, Morris TR, Dilda V, Naismith SL, et al. Assessing the utility of Freezing of Gait Questionnaires in Parkinson’s Disease. Parkinsonism Relat Disord. 2012;18(1):25-9.
319. Snijders AH, Nijkrake MJ, Bakker M, Munneke M, Wind C, Bloem BR. Clinimetrics of freezing of gait. Mov Disord. 2008;23 Suppl 2:S468-S74.
320. Snijders AH, Nonnekes J, Bloem BR. Recent advances in the assessment and treatment of falls in Parkinson’s disease. F1000 Med Rep. 2010;2:76.
321. Health UDo. The General Practice Physical Activity Questionnaire (GPPAQ). Beschikbaar via: https://wwwncbinlmnihgov/books/NBK51962/. Geraadpleegd 12 Jan 2006.
322. SHJ, Hendriks HJM, Bloem BR, Bredero-Cohen AB, Goede CJT de, Haaren M van, et al. KNGF-richtlijn ziekte van parkinson. Ned Tijdschr Fysiother. 2004;114 (Suppl)(3).
323. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738-50.
324. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997;26(5):353-7.
325. Nijkrake MJ, Keus SHJ, Quist-Anholts GWL, Bloem BR, Roode MH de, Lindeboom R, et al. Evaluation of a Patient Specific Index for Parkinson’s Disease (PSI-PD). European J Phys Rehabil Medicine. 2009;45(4):507-12.
326. Nieuwboer A, Herman T, Rochester L, Ehab Emil G, Giladi N. The new revised freezing of gait questionnaire, a reliable and valid instrument to measure freezing in Parkinson’s disease. Parkinsonism Relat Disord. 2008;14 (Suppl 1):S68.
327. Stack E, Ashburn A. Fall events described by people with Parkinson’s disease: implications for clinical interviewing and the research agenda. Physiother Res Int. 1999;4(3):190-200.
328. WHO. Global recommendations on physical activity for health. Beschikbaar via: wwwwhoint/dietphysicalactivity/publications/9789241599979/en/indexhtml. Geraadpleegd op 15 september 2010.
329. Kiresuk TJ, Smith A, Cardillo JE. Goal attainment scaling: applications, theory and measurement. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994.
330. Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil. 2009;23(4):362-70.
331. Young A, Chesson R. Goal attainment scaling as a method of measuring clinical outcome for children with learning disabilities. Br J Occ Ther. 1997;60(3):111-4.
332. Bouwens SF, Heugten CM van, Verhey FR. Review of goal attainment scaling as a useful outcome measure in psychogeriatric patients with cognitive disorders. Dement Geriatr Cogn Disord. 2008;26(6):528-40.
333. Turner-Stokes L, Williams H, Johnson J. Goal attainment scaling: does it provide added value as a person-centred measure for evaluation of outcome in Neuro Rehabil following acquired brain injury? J Rehabil Med. 2009;41(7):528-35.
334. Lannin N. Goal attainment scaling allows program evaluation of a home-based occupational therapy program. Occup Ther Health Care. 2003;17(1):43-54.
335. Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919-23.
336. Society AT. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-7.
337. Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123(2):387-98.
338. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
339. Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20(11):873-99.
340. Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28-M34.
341. Landers MR, Backlund A, Davenport J, Fortune J, Schuerman S, Altenburger P. Postural instability in idiopathic Parkinson’s disease: discriminating fallers from nonfallers based on standardized clinical measures. J Neurol Phys Ther. 2008;32(2):56-61.
342. Berg KO, Maki BE, Williams JI, Holliday PJ, Wood-Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. 1992;73(11):1073-80.
343. Groslambert A, Mahon AD. Perceived exertion: influence of age and cognitive development. Sports Med. 2006;36(11):911-28.
344. Shumway-Cook A, Woollacott M. Motor Control Theory and Applications. Baltimore: Williams and Wilkins; 1995. p. 323-4.
345. Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84(10):906-18.
346. Foreman KB, Addison O, Kim HS, Dibble LE. Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism Relat Disord. 2011;17(3):166-71.
347. Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing. 2005;34(6):614-9.
348. Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Phys Ther. 2005;85(10):1034-45.
349. Nieuwboer A, Weerdt W de, Dom R, Bogaerts K, Nuyens G. Development of an activity scale for individuals with advanced Parkinson disease: Reliability and “on-off” variability. Phys Ther. 2000;80(11):1087-96.
350. Arnadottir SA, Mercer VS. Effects of footwear on measurements of balance and gait in women between the ages of 65 and 93 years. Phys Ther. 2000;80(1):17-27.
351. Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42(4):323-31.
352. Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys Ther. 2013;93(2):158-67.
353. Charles C Thomas. (Ed.) The nine-hole peg test of finger hand coordination for the hemiplegic patient. 2e druk. Springfield (IL): 470-3; 1982.
354. Horak FB, Jacobs JV, Tran VK, Nutt JG. The push and release test: An improved clinical postural stability test for patients with Parkinson’s disease. Movement Disorders. 2004;19:S170-S.
355. Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-8.
356. Ashburn A, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson’s disease and the use of Falls Diaries to facilitate reporting. Disabil Rehabil. 2008;30(16):1205-12.
357. Latt MD, Lord SR, Morris JG, Fung VS. Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov Disord. 2009;24(9):1280-9.
358. Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLoSOne. 2009;4(5):e5521.
359. Camicioli R, Majumdar SR. Relationship between mild cognitive impairment and falls in older people with and without Parkinson’s disease: 1-Year Prospective Cohort Study. Gait Posture. 2010;32(1):87-91.
360. Smulders K, Nimwegen M van, Munneke M, Bloem BR, Kessels RP, Esselink RA. Involvement of specific executive functions in mobility in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(1):126-8.
361. Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116-24.
362. Amboni M, Cozzolino A, Longo K, Picillo M, Barone P. Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord. 2008;23(3):395-400.
363. Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329-42.
364. Duncan RP, Leddy AL, Cavanaugh JT, Dibble LE, Ellis TD, Ford MP, et al. Accuracy of fall prediction in Parkinson disease: six-month and 12-month prospective analyses. Parkinsons Dis. 2012;2012:237673.
365. Dibble LE, Lange M. Predicting falls in individuals with Parkinson disease: a reconsideration of clinical balance measures. J Neurol Phys Ther. 2006;30(2):60-7.
366. Dibble LE, Christensen J, Ballard DJ, Foreman KB. Diagnosis of fall risk in Parkinson disease: an analysis of individual and collective clinical balance test interpretation. Phys Ther. 2008;88(3):323-32.
367. Leddy AL, Crowner BE, Earhart GM. Functional gait assessment and balance evaluation system test: reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys Ther. 2011;91(1):102-13.
368. Leddy AL, Crowner BE, Earhart GM. Utility of the Mini-BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J Neurol Phys Ther. 2011;35(2):90-7.
369. King LA, Priest KC, Salarian A, Pierce D, Horak FB. Comparing the Mini-BESTest with the Berg Balance Scale to Evaluate Balance Disorders in Parkinson’s Disease. Parkinsons Dis. 2012;2012:375419.
370. Mak MK, Auyeung MM. The mini-BESTest can predict parkinsonian recurrent fallers: a 6-month prospective study. J Rehabil Med. 2013;45(6):565-71.
371. Duncan RP, Leddy AL, Earhart GM. Five times sit-to-stand test performance in Parkinson’s disease. Arch Phys Med Rehabil. 2011;92(9):1431-6.
372. Hendriks HJM, Oostendorp RAB, Bernards ATM, Ravensberg CD van, Heerkens YF, Nelson RM. The Diagnostic Process and Indication for Physiotherapy: A Prerequisite for Treatment and Outcome Evaluation. Phys Ther Reviews. 2000;5(1):29-47.
373. Bovend’Eerdt TJ, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: a practical guide. Clin Rehabil. 2009;23(4):352-61.
374. O’Brien M, Dodd KJ, Bilney B. A qualitative analysis of a progressive resistance exercise programme for people with Parkinson’s disease. Disabil Rehabil. 2008;30(18):1350-7.
375. Bodenheimer T, Davis C, Holman H. Helping patients adopt healthier behaviors. Clin Diabet. 2005;25(2):66-70.
376. Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88(6):733-46.
377. Dal Bello-Haas V, Klassen L, Sheppard MS, Metcalfe A. Psychometric Properties of Activity, Self-Efficacy, and Quality-of-Life Measures in Individuals with Parkinson Disease. Physiother Can. 2011;63(1):47-57.
378. Huang SL, Hsieh CL, Wu RM, Tai CH, Lin CH, Lu WS. Minimal detectable change of the timed Up & Go test and the dynamic gait index in people with Parkinson disease. Phys Ther. 2011;91(1):114-21.
379. Lim LIIK, Wegen EEH van, Goede CJT de, Jones D, Rochester L, Hetherington V, et al. Measuring gait and gait-related activities in Parkinson’s patients own home environment: a reliability, responsiveness and feasibility study. Parkinsonism Relat Disord. 2005;11(1):19-24.
380. Rae-Grant AD, Turner AP, Sloan A, Miller D, Hunziker J, Haselkorn JK. Self-management in neurological disorders: systematic review of the literature and potential interventions in multiple sclerosis care. J Rehabil Res Dev. 2011;48(9):1087-100.
381. Thompson DR, Chair SY, Chan SW, Astin F, Davidson PM, Ski CF. Motivational interviewing: a useful approach to improving cardiovascular health? J Clin Nurs. 2011;20(9-10):1236-44.
382. Teixeira PJ, Carraca EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: A systematic review. Int J Behav Nutr Phys Act. 2012;9(1):78.
383. Soderlund LL, Madson MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns. 2011;84(1):16-26.
384. Miller WR, Rollnick S. Meeting in the middle: motivational interviewing and self-determination theory. Int J Behav Nutr Phys Act. 2012;9:25.
385. Glasgow RE, Davis CL, Funnell MM, Beck A. Implementing practical interventions to support chronic illness self-management. Jt Comm J Qual Saf. 2003;29(11):563-74.
386. Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med. 2001;20(1):68-74.
387. Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009;76(2):174-80.
388. Battersby M, Von KM, Schaefer J, Davis C, Ludman E, Greene SM, et al. Twelve evidence-based principles for implementing self-management support in primary care. Jt Comm J Qual Patient Saf. 2010;36(12):561-70.
389. De Silva D. Helping people help themselves. Beschikbaar via: wwwhealthorguk/media_manager/public/75/publications_pdfs/Helping%20 people%20help%20themselvespdf. 2011.
390. Khalil H, Quinn L, Deursen R van, Martin R, Rosser A, Busse M. Adherence to use of a home-based exercise DVD in people with Huntington disease: participants’ perspectives. Phys Ther. 2012;92(1):69-82.
391. Lonsdale C, Hall AM, Williams GC, McDonough SM, Ntoumanis N, Murray A, et al. Communication style and exercise compliance in physiotherapy (CONNECT). A cluster randomized controlled trial to test a theory-based intervention to increase chronic low back pain patients’ adherence to physiotherapists’ recommendations: study rationale, design, and methods. BMC Musculoskelet Disord. 2012;13(1):104.
392. Morris ME, Martin CL, Schenkman ML. Striding out with Parkinson disease: evidence-based physical therapy for gait disorders. Phys Ther. 2010;90(2):280-8.
393. Schenkman M, Hall D, Kumar R, Kohrt WM. Endurance exercise training to improve economy of movement of people with Parkinson disease: three case reports. Phys Ther. 2008;88(1):63-76.
394. Speelman AD, Warrenburg BP van de, Nimwegen M van, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011;7(9):528-34.
395. McRae C, Sherry P, Roper K. Stress and family functioning among caregivers of persons with Parkinson’s disease. Parkinsonism Rel Disord. 1999;5:69-75.
396. Heath GW, Parra DC, Sarmiento OL, Andersen LB, Owen N, Goenka S, et al. Evidence-based intervention in physical activity: lessons from around the world. Lancet. 2012;380(9838):272-81.
397. Allen NE, Sherrington C, Suriyarachchi GD, Paul SS, Song J, Canning CG. Exercise and motor training in people with Parkinson’s disease: a systematic review of participant characteristics, intervention delivery, retention rates, adherence, and adverse events in clinical trials. Parkinsons Dis. 2012;2012:854328.
398. Speelman AD, Nimwegen M van, Bloem BR, Munneke M. Evaluation of implementation of the ParkFit program: A multifaceted intervention aimed to promote physical activity in patients with Parkinson’s disease. Physiotherapy. 2014 Jun;100(2):134-41. Epub 2013 21 Aug.
399. Achttien RJ, Staal JB, Merry AHH, Voort SSEM van der, Klaver RJ, Schoonewille S, et al. KNGF Guideline Cardiac Rehabilitation. Ned Tijdschr Fysiother. 2011;121(4):Suppl.
400. Association AH. Warning signs of heart attack, stroke and cardiac arrest.Beschikbaar via: www.heart org/HEARTORG/Conditions/Conditions_ UCM_305346_SubHomePage jsp. Geraadpleegd 20 januari 2012.
401. Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2013;9:CD002817.
402. Schmidt RA, Lee TD. Motor learning concepts and research methods. In: Schmidt RA, Lee TD, editors. Motor control and learning: A behavioral emphasis. 3rd ed. Champaign, IL: Human Kinetics; 1999. p. 263-84.
403. Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013;67:283-97.
404. Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron. 2011;72(3):469-76.
405. Nieuwboer A, Rochester L, Muncks L, Swinnen SP. Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord. 2009;15 Suppl 3:S53-8.
406. Rochester L, Baker K, Hetherington V, Jones D, Willems AM, Kwakkel G, et al. Evidence for motor learning in Parkinson’s disease: acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res. 2010;1319:103-11.
407. Abbruzzese G, Trompetto C, Marinelli L. The rationale for motor learning in Parkinson’s disease. Eur J Phys Rehabil Med. 2009;45(2):209-14.
408. Pendt LK, Reuter I, Muller H. Motor skill learning, retention, and control deficits in Parkinson’s disease. PLoS One. 2011;6(7):e21669.
409. Jessop RT, Horowicz C, Dibble LE. Motor learning and Parkinson disease: Refinement of movement velocity and endpoint excursion in a limits of stability balance task. Neurorehabil Neural Repair. 2006;20(4):459-67.
410. Soliveri P, Brown RG, Jahanshahi M, Marsden CD. Effect of practice on performance of a skilled motor task in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1992;55(6):454-60.
411. Swinnen SP, Steyvers M, Van Den Bergh L, Stelmach GE. Motor learning and Parkinson’s disease: refinement of within-limb and between-limb coordination as a result of practice. Behav Brain Res. 2000;111(1-2):45-59.
412. Chuma T. Rehabilitation for patients with Parkinson’s disease. J Neurol. 2007;254 Suppl 4:IV58-IV61.
413. Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage. 2007;35(1):222-33.
414. Hirsch MA, Farley BG. Exercise and neuroplasticity in persons living with Parkinson’s disease. Eur J Phys Rehabil Med. 2009;45(2):215-29.
415. Muslimovic D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson’s disease. Brain. 2007;130(Pt 11):2887-97.
416. Stephan MA, Meier B, Zaugg SW, Kaelin-Lang A. Motor sequence learning performance in Parkinson’s disease patients depends on the stage of disease. Brain Cogn. 2011;75(2):135-40.
417. Kwakkel G, Goede CJT de, Wegen EEH van. Impact of physical therapy for Parkinson’s disease: A critical review of the literature. Parkinsonism Relat Disord. 2007;13((Suppl.3)):S478-S87.
418. Onla-or S, Winstein CJ. Determining the optimal challenge point for motor skill learning in adults with moderately severe Parkinson’s disease. Neurorehabil Neural Repair. 2008;22(4):385-95.
419. Verschueren SM, Swinnen SP, Dom R, De Weerdt W. Interlimb coordination in patients with Parkinson’s disease: motor learning deficits and the importance of augmented information feedback. Exp Brain Res. 1997;113(3):497-508.
420. Krebs HI, Hogan N, Hening W, Adamovich SV, Poizner H. Procedural motor learning in Parkinson’s disease. Exp Brain Res. 2001;141(4):425-37.
421. Siegert RJ, Taylor KD, Weatherall M, Abernethy DA. Is implicit sequence learning impaired in Parkinson’s disease? A meta-analysis. Neuropsychology. 2006;20(4):490-5.
422. Mulder T. Motor imagery and action observation: cognitive tools for rehabilitation. J Neural Transm. 2007;114(10):1265-78.
423. Milton J, Small SL, Solodkin A. Imaging motor imagery: methodological issues related to expertise. Methods. 2008;45(4):336-41.
424. Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, Marsault C, et al. Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex. 2000;10(11):1093-104.
425. Zimmermann-Schlatter A, Schuster C, Puhan MA, Siekierka E, Steurer J. Efficacy of motor imagery in post-stroke rehabilitation: a systematic review. J Neuroeng Rehabil. 2008;5:8.
426. Celnik P, Webster B, Glasser DM, Cohen LG. Effects of action observation on physical training after stroke. Stroke. 2008;39(6):1814-20.
427. Ertelt D, Small S, Solodkin A, Dettmers C, McNamara A, Binkofski F, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36 Suppl 2:T164-T73.
428. Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14(1 Pt 2):S103-S9.
429. Avenanti A, Urgesi C. Understanding ‘what’ others do: mirror mechanisms play a crucial role in action perception. Soc Cogn Affect Neurosci. 2011;6(3):257-9.
430. Thobois S, Dominey PF, Decety J, Pollak PP, Gregoire MC, Le Bars PD, et al. Motor imagery in normal subjects and in asymmetrical Parkinson’s disease: a PET study. Neurology. 2000;55(7):996-1002.
431. Sackley C, Wade DT, Mant D, Atkinson JC, Yudkin P, Cardoso K, et al. Cluster randomized pilot controlled trial of an occupational therapy intervention for residents with stroke in UK care homes. Stroke. 2006;37(9):2336-41.
432. Behrman AL, Cauraugh JH, Light KE. Practice as an intervention to improve speeded motor performance and motor learning in Parkinson’s disease. J Neurol Sci. 2000;174(2):127-36.
433. Farley BG, Koshland GF. Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Experimental Brain Research. 2005;167(3):462-7.
434. Jobges M, Heuschkel G, Pretzel C, Illhardt C, Renner C, Hummelsheim H. Repetitive training of compensatory steps: a therapeutic approach for postural instability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(12):1682-7.
435. Lewis GN, Byblow WD, Walt SE. Stride length regulation in Parkinson’s disease: the use of extrinsic, visual cues. Brain. 2000;123 ( Pt 10):2077-90.
436. Platz T, Brown RG, Marsden CD. Training improves the speed of aimed movements in Parkinson’s disease. Brain. 1998;121 ( Pt 3):505-14.
437. Matinolli M, Korpelainen JT, Korpelainen R, Sotaniemi KA, Myllyla VV. Orthostatic hypotension, balance and falls in Parkinson’s disease. Mov Disord. 2009;24(5):745-51.
438. Wallace DM, Shafazand S, Carvalho DZ, Nahab FB, Sengun C, Russell A, et al. Sleep-related falling out of bed in Parkinson’s disease. J Clin Neurol. 2012;8(1):51-7.
439. Sanchez-Ferro A, ito-Leon J, Gomez-Esteban JC. The management of orthostatic hypotension in Parkinson’s disease. Front Neurol. 2013;4:64.
440. Cubo E, Moore CG, Leurgans S, Goetz CG. Wheeled and standard walkers in Parkinson’s disease patients with gait freezing. Parkinsonism Relat Disord. 2003;10(1):9-14.
441. Krebs DE, Scarborough DM, McGibbon CA. Functional vs. strength training in disabled elderly outpatients. Am J Phys Med Rehabil. 2007;86(2):93-103.
442. Vreede PL de, Samson MM, Meeteren NL van, Duursma SA, Verhaar HJ. Functional-task exercise versus resistance strength exercise to improve daily function in older women: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(1):2-10.
443. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687-708.
444. Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson’s disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33(1):14-26.
445. Lima LO, Scianni A, Rodrigues-de-Paula F. Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson’s disease: a systematic review. J Physiother. 2013;59(1):7-13.
446. University As, Institute NC. Compendium of Physical Activities. Beschikbaar via: https://sitesgooglecom/site/compendiumofphysicalactivities.
447. Goss FL, Robertson RJ, Haile L, Nagle EF, Metz KF, Kim K. Use of ratings of perceived exertion to anticipate treadmill test termination in patients taking beta-blockers. Percept Mot Skills. 2011;112(1):310-8.
448. Gallo P, Garber C. Parkinson’s Disease: A Comprehensive Approach to Exercise Prescription for the Health Fitness Professional. ACSM’s Health Fitness J. 2011;15(4):8-17.
449. Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005(1):Cd004017.
450. Stanley RK, Protas EJ, Jankovic J. Exercise performance in those having Parkinson’s disease and healthy normals. Med Sci Sports Exerc. 1999;31(6):761-6.
451. Speelman AD, Groothuis JT, Nimwegen M van, Scheer ES van der, Borm GF, Bloem BR, et al. Cardiovascular responses during a submaximal exercise test in patients with Parkinson’s disease. J Parkinsons Dis. 2012;2(3):241-7.
452. McGinley JL, Martin C, Huxham FE, Menz HB, Danoudis M, Murphy AT, et al. Feasibility, safety, and compliance in a randomized controlled trial of physical therapy for Parkinson’s disease. Parkinsons Dis. 2012;2012:795294.
453. Nieuwboer A. Cueing for freezing of gait in patients with Parkinson’s disease: a rehabilitation perspective. Mov Disord. 2008;23 Suppl 2:S475-S81.
454. Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage. 2003;19(3):764-76.
455. Brown RG, Marsden CD. Internal versus external cues and the control of attention in Parkinson’s disease. Brain. 1988;111 ( Pt 2):323-45.
456. Brown RG, Marsden CD. An investigation of the phenomenon of “set” in Parkinson’s disease. Mov Disord. 1988;3(2):152-61.
457. Rochester L, Hetherington V, Jones D, Nieuwboer A, Willems AM, Kwakkel G, et al. The effect of external rhythmic cues (auditory and visual) on walking during a functional task in homes of people with Parkinson’s disease. Arch Phys Med Rehabil. 2005;86(5):999-1006.
458. Rochester L, Nieuwboer A, Baker K, Hetherington V, Willems AM, Chavret F, et al. The attentional cost of external rhythmical cues and their impact on gait in Parkinson’s disease: effect of cue modality and task complexity. J Neural Transm. 2007;114(10):1243-8.
459. Rochester L, Burn DJ, Woods G, Godwin J, Nieuwboer A. Does auditory rhythmical cueing improve gait in people with Parkinson’s disease and cognitive impairment? A feasibility study. Mov Disord. 2009;24(6):839-45.
460. Nieuwboer A, Rochester L, Jones D. Cueing gait and gait-related mobility in patients with Parkinson’s disease. Top Geriatr Rehab. 2008;24:151-65.
461. Jiang Y, Norman KE. Effects of visual and auditory cues on gait initiation in people with Parkinson’s disease. Clin Rehab. 2006;20(1):36-45.
462. Dibble LE, Nicholson DE, Shultz B, MacWilliams BA, Marcus RL, Moncur C. Sensory cueing effects on maximal speed gait initiation in persons with Parkinson’s disease and healthy elders. Gait Posture. 2004;19(3):215-25.
463. Praamstra P, Stegeman DF, Cools AR, Horstink MW. Reliance on external cues for movement initiation in Parkinson’s disease. Evidence from movement-related potentials. Brain. 1998;121 ( Pt 1):167-77.
464. Willems AM, Nieuwboer A, Chavret F, Desloovere K, Dom R, Rochester L, et al. The use of rhythmic auditory cues to influence gait in patients with Parkinson’s disease, the differential effect for freezers and non-freezers, an explorative study. Disabili Rehabil. 2006;28(11):721-8.
465. Dreu MJ de, Wilk AS van der, Poppe E, Kwakkel G, Wegen EE van. Rehabilitation, exercise therapy and music in patients with Parkinson’s disease: a meta-analysis of the effects of music-based movement therapy on walking ability, balance and quality of life. Parkinsonism Relat Disord. 2012;18 Suppl 1:S114-S9.
466. Volpe D, Signorini M, Marchetto A, Lynch T, Morris ME. A comparison of Irish set dancing and exercises for people with Parkinson’s disease: a phase II feasibility study. BMC Geriatr. 2013;13:54.
467. Brauer SG, Woollacott MH, Lamont R, Clewett S, O’Sullivan J, Silburn P, et al. Single and dual task gait training in people with Parkinson’s disease: a protocol for a randomised controlled trial. BMC Neurol. 2011;11:90.
468. Strouwen C, Molenaar EA, Keus SH, Munks L, Munneke M, Vandenberghe W, et al. Protocol for a randomized comparison of integrated versus consecutive dual task practice in Parkinson’s disease: the DUALITY trial. BMC Neurol. 2014;14:61.
469. Canning CG. The effect of attention on walking performance under dual-task conditions in individuals with Parkinson’s disease. Aust J Physiother. 2003;49(4 (Suppl)):S8.
470. Kelly VE, Eusterbrock AJ, Shumway-Cook A. The effects of instructions on dual-task walking and cognitive task performance in people with Parkinson’s disease. Parkinsons Dis. 2012;2012:671261.
471. Yogev-Seligmann G, Rotem-Galili Y, Dickstein R, Giladi N, Hausdorff JM. Effects of explicit prioritization on dual task walking in patients with Parkinson’s disease. Gait Posture. 2012;35(4):641-6.
472. Canning CG, Ada L, Woodhouse E. Multiple-task walking training in people with mild to moderate Parkinson’s disease: a pilot study. Clin Rehabil. 2008;22(3):226-33.
473. Galletly R, Brauer SG. Does the type of concurrent task affect preferred and cued gait in people with Parkinson’s disease? Aust J Physiother. 2005;51(3):175-80.
474. Mak MK, Yu L, Hui-Chan CW. The immediate effect of a novel audio-visual cueing strategy (simulated traffic lights) on dual-task walking in people with Parkinson’s disease. Eur J Phys Rehabil Med. 2013;49(2):153-9.
475. Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? J Gerontol A Biol Sci Med Sci. 2011;66(2):234-40.
476. Baker K, Rochester L, Nieuwboer A. The immediate effect of attentional, auditory, and a combined cue strategy on gait during single and dual tasks in Parkinson’s disease. Arch Phys Med Rehabil. 2007;88(12):1593- 600.
477. Earhart GM, Williams AJ. Treadmill training for individuals with Parkinson disease. Phys Ther. 2012;92(7):893-7.
478. Mehrholz J, Friis R, Kugler J, Twork S, Storch A, Pohl M. Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst Rev. 2010(1):CD007830.
479. Pelosin E, Bove M, Ruggeri P, Avanzino L, Abbruzzese G. Reduction of Bradykinesia of Finger Movements by a Single Session of Action Observation in Parkinson Disease. Neurorehabil Neural Repair. 2013.
480. Sitja RM, Rigau CD, Fort VA, Santoyo MC, Figuls M, Romero-Rodriguez D, et al. Whole-body vibration training for patients with neurodegenerative disease. Cochrane Database Syst Rev. 2012;2:CD009097.
481. Muir J, Kiel DP, Rubin CT. Safety and severity of accelerations delivered from whole body vibration exercise devices to standing adults. J Sci Med Sport. 2013;16(6):526-31.
482. Butler D, Moseley L. Explain pain. Aidelaide, South Australia: Noigroup Publications; 2003.
483. Saleem AF, Sapienza CM, Rosenbek JC, Musson ND, Okun MS. The effects of expiratory muscle strength training on pharyngeal swallowing in patients with idiopathic Parkinson’s disease. Neurology. 2005;64(6):A397-A.
484. Bott J, Blumenthal S, Buxton M, Ellum S, Falconer C, Garrod R, et al. Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax. 2009;64 Suppl 1:i1-51.
485. Kang SW, Bach JR. Maximum insufflation capacity. Chest. 2000;118(1):61-5. 486.Chatwin M, Ross E, Hart N, Nickol AH, Polkey MI, Simonds AK. Cough augmentation with mechanical insufflation/exsufflation in patients with neuromuscular weakness. Eur Respir J. 2003;21(3):502-8.
486. Chatwin M, Ross E, Hart N, Nickol AH, Polkey MI, Simonds AK. Cough augmentation with mechanical insuffl ation/exsuffl ation in patients with neuromuscular weakness. Eur Respir J. 2003;21(3):502-8.
487. Trebbia G, Lacombe M, Fermanian C, Falaize L, Lejaille M, Louis A, et al. Cough determinants in patients with neuromuscular disease. Respir Physiol Neurobiol. 2005;146(2-3):291-300.
488. Benditt JO. Management of pulmonary complications in neuromuscular disease. Phys Med Rehabil Clin N Am. 1998;9(1):167-85.
489. Bach JR. Mechanical insufflation-exsufflation. Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest. 1993;104(5):1553-62.
490. Reuter I, Mehnert S, Sammer G, Oechsner M, Engelhardt M. Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in Parkinson’s disease. J Aging Res. 2012;2012:235765.
491. Eijk M van der, Faber MJ, Aarts JW, Kremer JA, Munneke M, Bloem BR. Using online health communities to deliver patient-centered care to people with chronic conditions. JMed InternetRes. 2013;15(6):e115.
492. Weerkamp NJ, Tissingh G, Poels PJ, Zuidema SU, Munneke M, Koopmans RT, et al. Parkinson disease in long term care facilities: a review of the literature. J Am Med Dir Assoc. 2014;15(2):90-4.
